In chemistry, calculating the percentage abundance of a particular isotope is an essential concept that helps determine the atomic mass and properties of elements. By learning how to calculate the percentage abundance of an isotope, you can get a better understanding of how atoms are structured and how they interact with other elements.
Many students find it challenging to calculate the percentage abundance of isotopes because it involves mathematical formulas and complex concepts. But do not worry. In this article, we will break down the process of how to calculate percentage abundance chem and provide helpful tips to make the process easier.
The percentage abundance of an isotope is calculated by dividing the mass of the isotope by the mass of the element and then multiplying by 100. This calculation can be used to determine the percentage of each isotope present in a given sample.
To begin calculating the percentage abundance, you first need to determine the atomic mass of the element by multiplying the mass of each isotope by its percentage abundance, summing up the resulting values, and then dividing by 100. After determining the atomic mass, you can calculate the percentage abundance of each isotope.
Personal Experience
When I first learned about the concept of percentage abundance in chemistry, I found it challenging to understand at first. However, after practicing multiple problems and getting a better understanding of the formulas used, I soon realized that calculating percentage abundance could be much easier than I thought. The key is to break down each step and understand the concepts behind each formula.
Tips for Calculating Percentage Abundance
Here are some helpful tips for calculating the percentage abundance of isotopes:
- Write down all the information provided in the problem, including the mass of each isotope and the atomic mass of the element.
- Convert the percentages into decimal form for ease of calculation.
- Use the formula: (mass of isotope / atomic mass of element) * 100 to calculate the percentage abundance of each isotope.
- Remember to round your answer to the nearest whole number if necessary.
Understanding Atomic Mass Units
In chemistry, we measure the masses of atoms using atomic mass units (amu). One amu equates to one-twelfth the mass of one carbon-12 atom. When working with isotopes, the atomic mass of an element is not a whole number due to each isotope’s different mass number. The atomic mass of an element is calculated as a weighted average of the masses of each isotope, taking into account its percentage abundance.
Examples of Calculating Percentage Abundance
Let’s look at an example of calculating percentage abundance. Suppose we have an element with two isotopes, A and B. The mass of isotope A is 4 amu, and its abundance is 25%. The mass of isotope B is 6 amu, and its abundance is 75%. To calculate the atomic mass of the element, we use the formula:
(4 amu * 0.25) + (6 amu * 0.75) = 5.5 amu
Now that we know the atomic mass, we can calculate the percentage abundance of each isotope using the formula:
Percentage abundance of isotope A = (4 amu / 5.5 amu) * 100 = 72.7%
Percentage abundance of isotope B = (6 amu / 5.5 amu) * 100 = 27.3%
FAQs
How do you calculate percentage abundance?
To calculate percentage abundance, divide the mass of the isotope by the atomic mass of the element and multiply by 100.
How do you calculate the atomic mass of an element?
To calculate the atomic mass of an element, multiply the mass of each isotope by its percentage abundance, sum up the resulting values, and divide by 100.
What is the purpose of calculating percentage abundance?
By calculating percentage abundance, you can determine the amount of each isotope present in a sample and the atomic mass of the element.
What are atomic mass units?
Atomic mass units (amu) are used to measure the masses of atoms. One amu is equal to one-twelfth the mass of one carbon-12 atom.
Conclusion
Calculating the percentage abundance of isotopes in chemistry is an important concept that helps us understand the atomic structure of elements. By following the formula and tips provided in this article, you can easily calculate the percentage abundance of isotopes and determine the atomic mass of an element. Remember to practice multiple problems for mastery of the concept and use online resources to supplement your learning. Happy calculating!
Gallery
How To Calculate Percent Abundance In Chemistry

Photo Credit by: bing.com / abundance percent calculate chemistry
Calculating Percent Abundance Of Isotopes Worksheet – Worksheet

Photo Credit by: bing.com / abundance percent calculating isotopes isotope find each equation amu worksheet
What Is Abundance In Chemistry – Tutordale.com
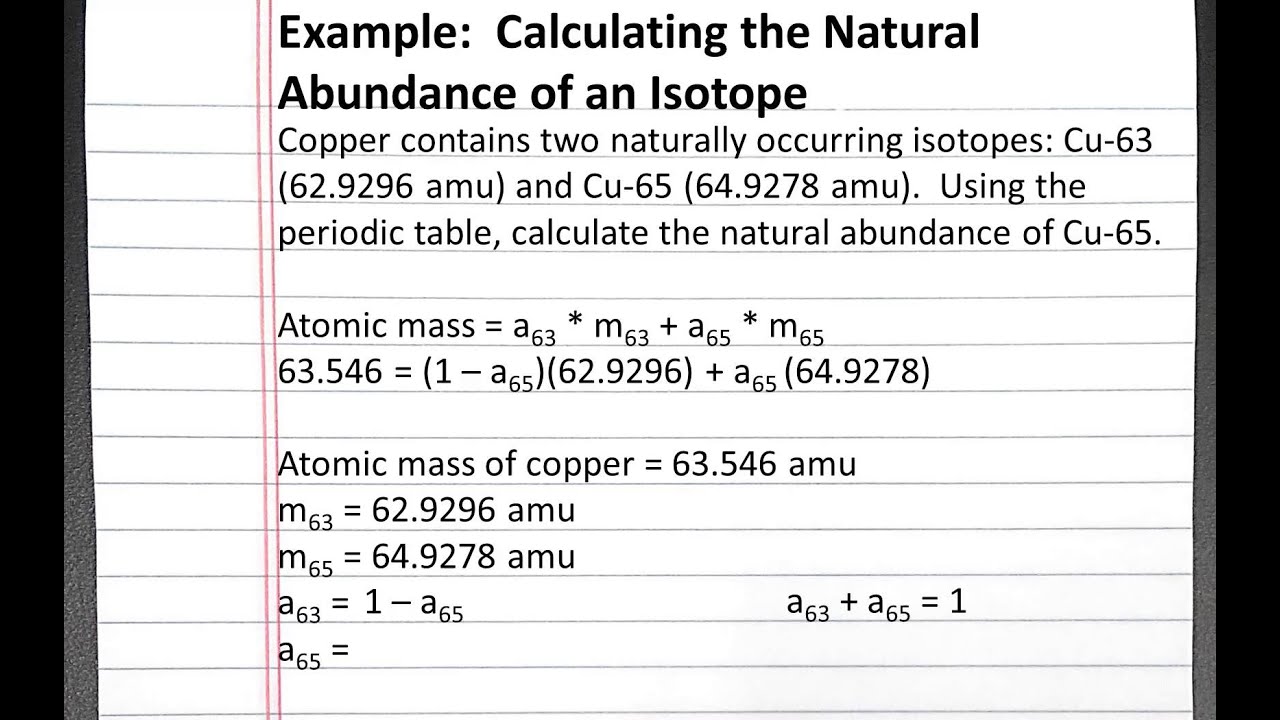
Photo Credit by: bing.com /
How To Calculate Percent Abundance Of Each Isotope
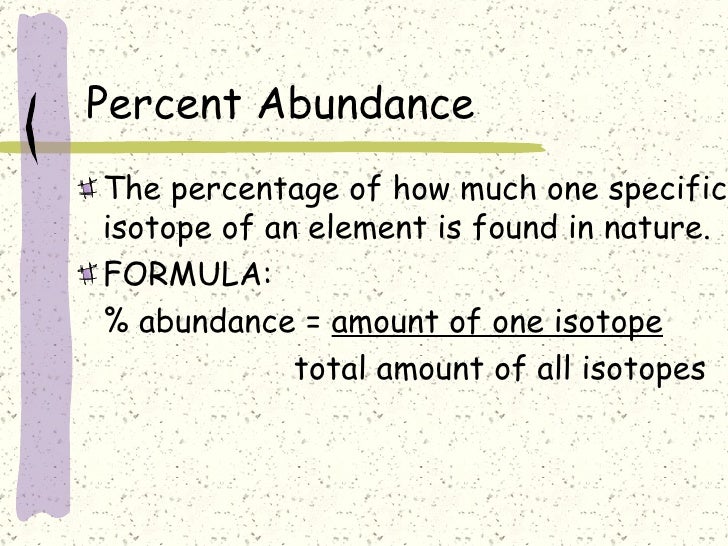
Photo Credit by: bing.com / abundance percent isotope s05 calculate
Calculating Percentage Abundance Of Each Isotope – YouTube

Photo Credit by: bing.com / abundance calculating isotope