Are you struggling with calculating percentage abundance in chemistry? Don’t worry, you’re not alone. Many students find this concept challenging, but it’s an essential skill that you’ll need to master to succeed in chemistry. In this blog post, we’ll break down how to calculate percentage abundance and provide helpful tips to make the process easier.
When trying to calculate the percentage abundance of an isotope, students often struggle with understanding what information they need and how to use it. It can also be challenging to know which formula to use and how to apply it correctly. Fortunately, with a bit of practice and understanding, you’ll be able to tackle these challenges and improve your understanding of percentage abundance.
The first step to calculating the percentage abundance of an isotope is to determine the number of atoms for each isotope in a sample. Once you have this information, you can use the following formula:

Where:
- X = percentage abundance of the first isotope
- Y = percentage abundance of the second isotope
- A = atomic mass of the first isotope
- B = atomic mass of the second isotope
By plugging in the values for each variable, you’ll be able to calculate the percentage abundance of each isotope in the sample.
My Personal Experience with Calculating Percentage Abundance
When I was first introduced to the concept of percentage abundance in chemistry, I found it challenging to understand what information was necessary to calculate it. However, after talking with my professor and practicing with different examples, I began to grasp the concept better.
One trick that helped me was creating a chart with the number of atoms for each isotope in a sample. From there, I could easily plug in the values into the formula and calculate the percentage abundance. If you’re struggling with this concept, I recommend trying this method to see if it works for you.
Tips for Calculating Percentage Abundance
To improve your skills in calculating percentage abundance, we’ve compiled a few tips to help make the process easier:
- Create a chart with the number of atoms for each isotope in a sample
- Make sure you use the correct atomic masses for each isotope
- Double-check your calculations to avoid errors
- Practice with different examples to improve your understanding
Understanding Isotopes
To calculate percentage abundance, it’s essential to understand what isotopes are. In chemistry, isotopes are atoms of the same element with different numbers of neutrons. These isotopes have slightly different atomic masses, which is what makes calculating percentage abundance possible.
For example, carbon has three naturally occurring isotopes: carbon-12, carbon-13, and carbon-14. Carbon-12 is the most abundant isotope, and carbon-14 is the least abundant. By understanding the number of atoms for each isotope in a sample of carbon, you can calculate the percentage abundance of each isotope.
Atomic Masses
Another essential concept to understand when calculating percentage abundance is atomic masses. The atomic mass of an element is the sum of the masses of its isotopes, weighted by their abundance.
For example, if you have a sample of carbon with two isotopes: carbon-12 and carbon-13, you would calculate the atomic mass as follows:

Where:
- X = percentage abundance of carbon-12
- Y = percentage abundance of carbon-13
- A = atomic mass of carbon-12
- B = atomic mass of carbon-13
By calculating the atomic mass, you’ll be able to determine the abundance of each isotope and calculate the percentage abundance successfully.
Practice Makes Perfect
Like any skill, the more you practice calculating percentage abundance, the better you’ll become. We recommend working on several different examples until you feel comfortable applying the formula and understanding the concept of isotopes and atomic masses.
Question and Answer
Q: What is percentage abundance?
A: Percentage abundance is the percentage of each isotope present in a sample of an element.
Q: What is the formula for calculating percentage abundance?
A: The formula for calculating percentage abundance is:

Q: How do isotopes impact percentage abundance?
A: Isotopes impact percentage abundance because they have different atomic masses, which allows us to calculate the percentage abundance of each isotope in a sample.
Q: How can you double-check your calculations for percentage abundance?
A: You can double-check your calculations for percentage abundance by ensuring you’re using the correct atomic masses for each isotope and verifying that the percentages add up to 100%.
Conclusion of how to calculate percentage abundance
Calculating percentage abundance in chemistry can be challenging, but with practice and a solid understanding of isotopes and atomic masses, you’ll be able to master this skill. Remember to create charts, double-check your calculations, and practice with different examples. By doing so, you’ll be able to confidently calculate percentage abundance and improve your chemistry skills.
Gallery
How To Calculate Percent Abundance In Chemistry

Photo Credit by: bing.com / abundance percent calculate chemistry
Calculating Percent Abundance Rubidium Rb Isotopes Example – YouTube

Photo Credit by: bing.com / abundance percent isotopes rubidium calculating example rb
Calculating Percentage Abundance Of Each Isotope – YouTube

Photo Credit by: bing.com / abundance calculating isotope
ShowMe – Percent Abundance
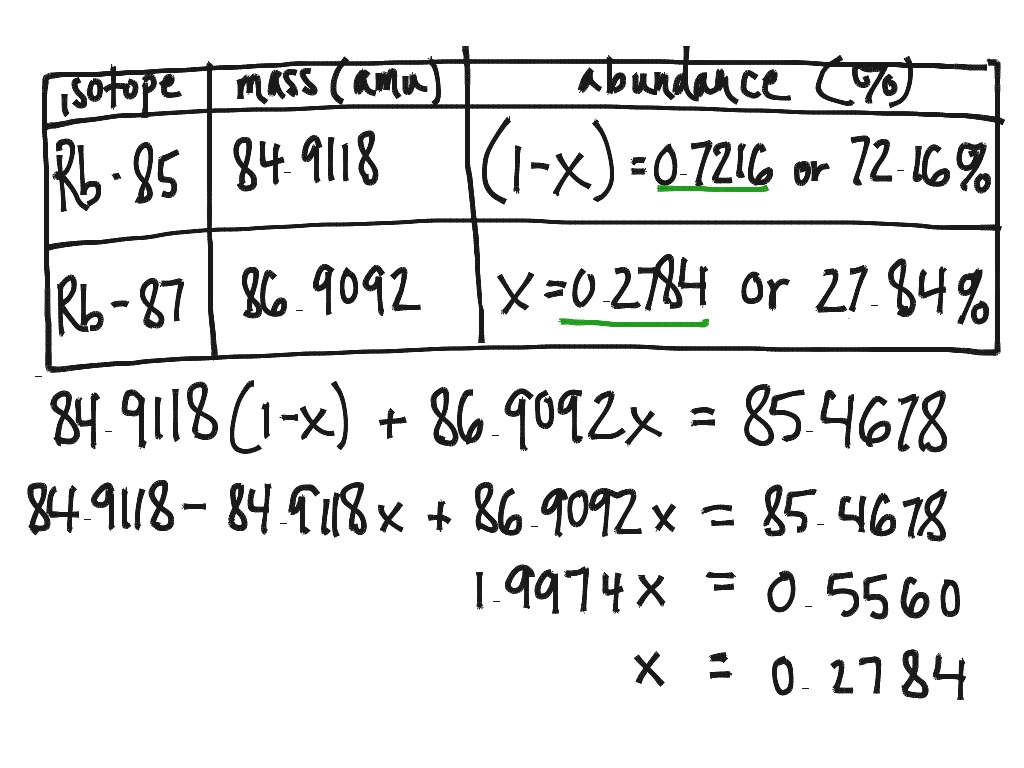
Photo Credit by: bing.com / isotopes calculating
Solved: My Professor Provided A Key To Our Quiz From Last | Chegg.com
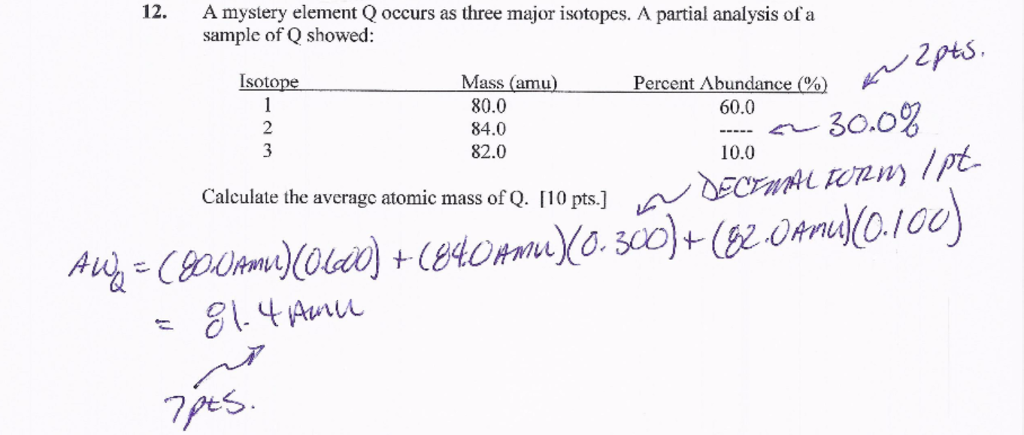
Photo Credit by: bing.com / abundance isotopes quiz atomic solved