Are you struggling to calculate percentage atom economy? Do you find yourself lost in the sea of chemistry equations and just can’t seem to figure it out? Look no further, as we provide a comprehensive guide on how to calculate percentage atom economy.
The Struggles of Calculating Percentage Atom Economy
For many students or professionals in the chemistry field, calculating percentage atom economy can be a challenge. The concept itself may seem straightforward, but the equations and steps involved can be daunting for newcomers. This can lead to frustration and a lack of understanding, which can ultimately hinder one’s progress in their studies or work.
Answering the Target of How to Calculate Percentage Atom Economy
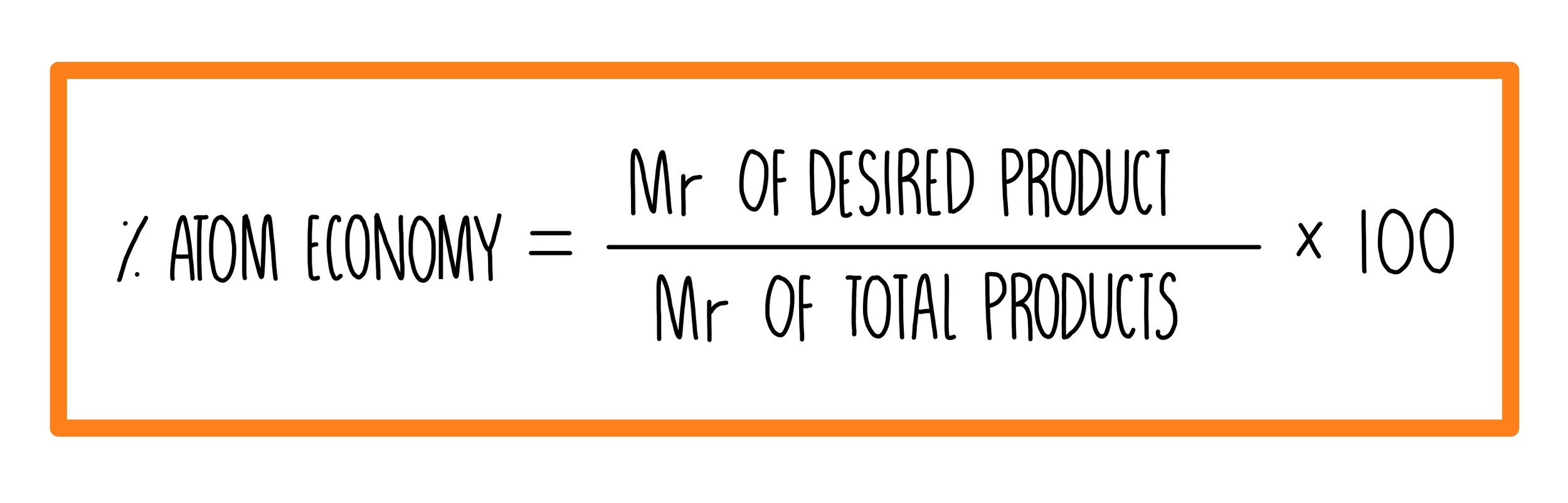
Put simply, calculating percentage atom economy is a way to measure the efficiency of a chemical reaction. It is expressed as the percentage of the reactants that successfully become the desired product, taking into account the masses of the atoms involved. This information can be useful in determining the environmental impact of a particular reaction, as well as determining the profitability for industrial-scale reactions.
Summary of Main Points
To calculate percentage atom economy, one must first determine the mass of the desired product and the masses of all the atoms present in the reactants. From there, one can calculate the maximum theoretical yield of the product, and then the actual yield once the reaction takes place. The percentage atom economy can then be calculated using these values. It is important to consider the environmental impact and profitability when analyzing the results.
How to Calculate Percentage Atom Economy – Step by Step
As mentioned earlier, to calculate percentage atom economy, one must first determine the mass of the desired product and the masses of all the atoms present in the reactants. From there, follow these steps to get your percentage atom economy:
- Calculate the maximum theoretical yield of the product, based on the masses of atoms in the reactants
- Perform the actual reaction and determine the actual yield
- Calculate the percentage yield by dividing actual yield by theoretical yield and multiplying by 100
- Calculate percentage atom economy by dividing the actual yield mass by the total mass of the atoms in the reactants and multiplying by 100
It is important to note that the percentage atom economy should ideally be as high as possible, as this indicates a more efficient use of the reactants and less waste being produced.
Going Deeper into the Explanation
Let’s take a closer look at calculating percentage atom economy using an example:
In this reaction, the desired product is water, with hydrogen and oxygen gas being the reactants. The masses of each atom are as follows:
- Hydrogen – 2g
- Oxygen – 32g
Using these values, we can calculate the maximum theoretical yield of water to be 18g. Now let’s say we perform the reaction and obtain an actual yield of 12g. To calculate the percentage yield, we divide the actual yield (12g) by the theoretical yield (18g) and multiply by 100, resulting in 66.67%. To calculate the percentage atom economy, we divide the actual yield mass (12g) by the total mass of the atoms in the reactants (34g) and multiply by 100, resulting in 35.29%. This means that only 35.29% of the mass of the reactants was utilized in creating the desired product, indicating a relatively inefficient reaction.
Making Sense of the Calculations
To fully understand the significance of the calculations involved in percentage atom economy, it is important to consider the environmental impact and profitability of the reaction. In the example above, a low percentage atom economy would mean that a significant amount of waste is being produced, which can potentially harm the environment. It can also indicate a less profitable reaction, as a lower yield means less product to sell.
Optimizing Percentage Atom Economy
Now that we know how to calculate percentage atom economy, how can we optimize it? There are several ways to improve the efficiency of a reaction, such as:

- Using catalysts to reduce reaction time and minimize the amount of energy required
- Using renewable or green reactants to reduce waste and environmental impact
- Designing more efficient reactors, such as those with better heat transfer or increased surface area for reactants to make contact
Further Explanation on Optimization
By implementing these strategies, we can aim to achieve a higher percentage atom economy, indicating a more efficient and profitable reaction. It is important to always consider the environmental and economic impact when designing and analyzing chemical reactions.
Personal Take on Optimization
As a chemistry student, I have seen firsthand the importance of considering percentage atom economy in designing efficient and sustainable reactions. By optimizing the efficiency of a reaction, we not only reduce waste and environmental harm but also increase profitability and potential for future advancements.
Question and Answer
Here are some frequently asked questions about how to calculate percentage atom economy:
What is a good percentage atom economy?
There is no specific percentage that is considered “good,” as it can vary depending on the specific reaction and the desired product. However, a higher percentage atom economy is generally considered more desirable, as it indicates a more efficient use of reactants with less waste produced.
Why is percentage atom economy important?
Percentage atom economy is important as it allows us to measure the efficiency of a chemical reaction and consider the environmental impact and profitability. By optimizing percentage atom economy, we can aim for more sustainable and profitable reactions.
What factors can affect percentage atom economy?
Several factors can affect percentage atom economy, such as reaction time, temperature, catalysts, and reactant quality. By optimizing these factors, we can aim for a higher percentage atom economy.
What other calculations can be useful in measuring reaction efficiency?
Other useful calculations include yield, which measures the amount of product obtained from a reaction, and selectivity, which measures the amount of desired product obtained compared to unwanted byproducts.
Conclusion of How to Calculate Percentage Atom Economy
Calculating percentage atom economy may seem daunting at first, but with practice and understanding, it can be a useful tool for measuring and optimizing the efficiency of chemical reactions. By considering the environmental and economic impact, we can strive for more sustainable and profitable reactions.
Gallery
27+ How To Calculate Atom Economy – LudovicTawsif

Photo Credit by: bing.com /
Percentage Yield And Atom Economy (AQA) — The Science Hive

Photo Credit by: bing.com /
Atom Economy Calculation – YouTube

Photo Credit by: bing.com / atom economy calculation
Calculating The Percentage Atom Economy Of A Reaction – Video & Lesson

Photo Credit by: bing.com / atom economy equation percentage reaction percent calculating study steps
How To Calculate Atom Economy – YouTube
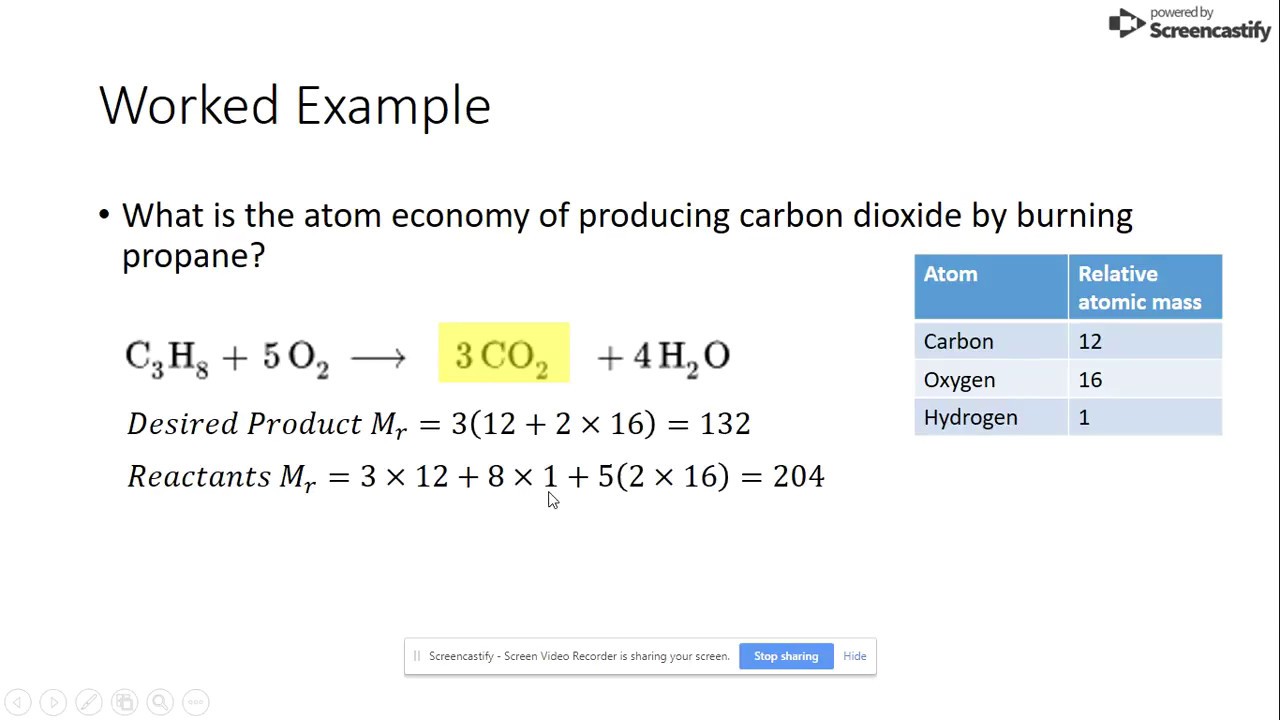
Photo Credit by: bing.com / atom economy calculate