Are you struggling to understand how to calculate percentage atom economy gcse? Don’t worry, you’re not alone. Many students find this topic challenging, but with the right guidance, it can be simple and even enjoyable! In this article, we’ll break down the steps to calculate percentage atom economy gcse and provide you with some tips and tricks to make the process easier.
Calculating percentage atom economy gcse can be a bit confusing, especially when you’re first getting started. It’s important to have a good understanding of the concept and the calculations involved in order to be successful. Once you have a grasp of the basics, however, you’ll find that it’s a straightforward process that gets easier with practice.
So, what exactly is percentage atom economy gcse? In simple terms, it is a measure of how efficiently reactant atoms are used to form desired products. To calculate it, you need to know the balanced chemical equation for the reaction, as well as the molecular masses of the reactants and products.
Before we dive into the details of how to calculate percentage atom economy gcse, let’s summarize the main points. To calculate percentage atom economy gcse, you need to know the balanced chemical equation, as well as the molecular masses of the reactants and products. Once you have this information, you can use a simple formula to calculate the percentage atom economy. With practice, this process will become easier and more intuitive.
Calculating Percentage Atom Economy GCSE
When learning how to calculate percentage atom economy gcse, it’s helpful to have a personal experience to relate to. Consider the following scenario:

Suppose you are conducting a chemical reaction in which the reactants have a combined molecular mass of 100 g, and the products have a molecular mass of 80 g. To calculate the percentage atom economy, you would use the following formula:
Percentage Atom Economy = (Total Mass of Desired Product / Total Mass of Reactants) x 100
In this case, the total mass of the desired product is 80 g, and the total mass of the reactants is 100 g. Plugging these values into the formula, we get:
Percentage Atom Economy = (80 / 100) x 100 = 80%
So, the percentage atom economy for this reaction is 80%. This means that 80% of the reactant atoms were used to form the desired product.
Tips and Tricks for Calculating Percentage Atom Economy GCSE
Now that you know how to calculate percentage atom economy gcse, it’s important to keep a few tips and tricks in mind to make the process as easy as possible. Here are a few things to keep in mind:
- Always start with a balanced chemical equation.
- Make sure you know the molecular masses of all reactants and products.
- Pay attention to units – make sure they are all in the same system (e.g. grams).
- Double-check your calculations to avoid errors.
Why is Percentage Atom Economy Important?
Percentage atom economy is an important concept in chemistry because it allows us to measure the efficiency of a chemical reaction. In practical terms, this means that we can design reactions that use reactants more efficiently, leading to less waste and a more sustainable chemical industry.
How is Percentage Atom Economy Used in Industry?
In industry, percentage atom economy is used to design reactions that are more efficient and create less waste. This can help reduce costs and environmental impact, as well as increase profits. By carefully considering atom economy when designing chemical processes, companies can create more sustainable and profitable operations.
Frequently Asked Questions
What is Atom Economy?
Atom economy is a measure of the efficiency of a chemical process in terms of the number of atoms of starting materials that end up in the desired product. A higher atom economy means that more starting material atoms are incorporated into the final product, resulting in less waste.
What is the Formula for Percentage Atom Economy?
The formula for calculating percentage atom economy is:
Percentage Atom Economy = (Total Mass of Desired Product / Total Mass of Reactants) x 100
Why is Atom Economy Important?
Atom economy is important because it helps to drive sustainability in the chemical industry. Reactions with high atom economy result in less waste and a more efficient use of resources, which can help reduce costs and minimize environmental impact.
What is the Difference Between Atom Economy and Yield?
Atom economy and yield are related but distinct concepts. Yield refers to the amount of desired product formed in a chemical reaction, expressed as a percentage of the theoretical maximum. Atom economy, on the other hand, takes into account the efficiency of the reaction in terms of how many of the starting material atoms ended up in the final product.
Conclusion of How to Calculate Percentage Atom Economy GCSE
Calculating percentage atom economy gcse can be challenging at first, but with practice and a good understanding of the concept, it becomes easier over time. By following the tips and tricks outlined in this article, you can make the process as streamlined as possible, allowing you to focus on the more interesting aspects of chemistry. Remember that atom economy is an important concept in the chemical industry, and by designing reactions with high atom economy, we can create more sustainable and profitable operations.
Gallery
Atom Economy Calculation – YouTube

Photo Credit by: bing.com / atom economy calculation
Percentage Yield And Atom Economy (AQA) — The Science Hive
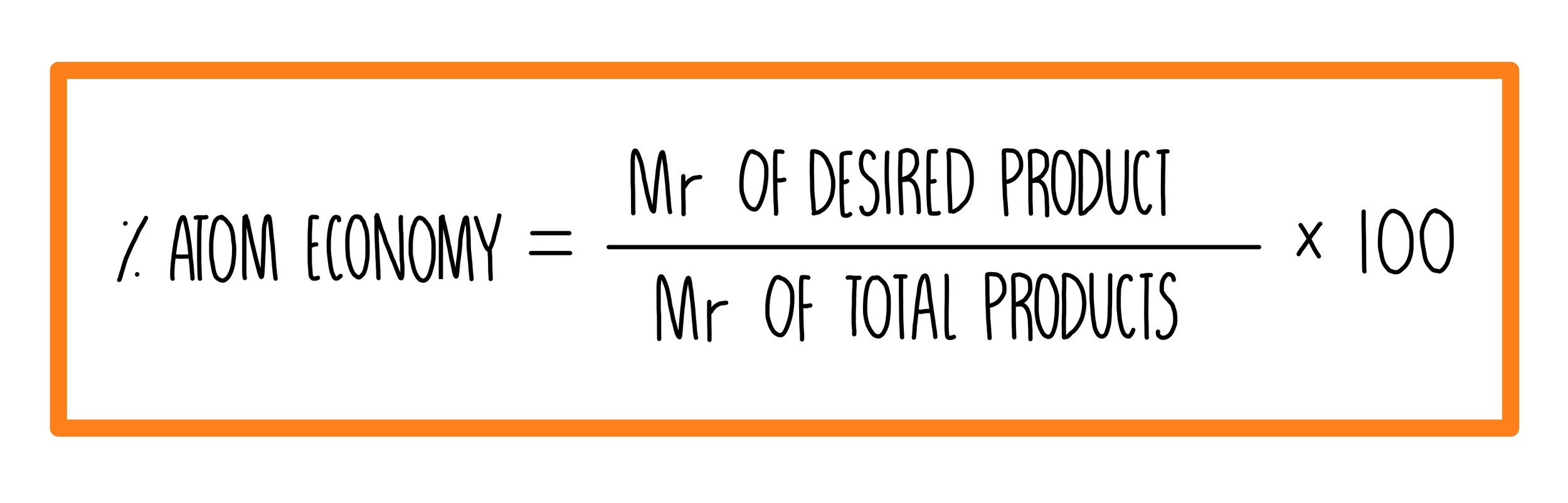
Photo Credit by: bing.com /
Atom Economy – Yield

Photo Credit by: bing.com / atom economy phenol yield equation calculating methods produced variety been
Percentage Yield And Atom Economy (GCSE And A Level) | Teaching Resources

Photo Credit by: bing.com / atom gcse
GCSE Science Chemistry (9-1 Triple) Atom Economy – YouTube

Photo Credit by: bing.com / economy atom gcse chemistry science