If you’re studying chemistry, there’s a good chance that you’ll need to know how to calculate percentage by mass. This calculation is used to determine the amount of a particular substance in a larger sample based on its weight. It may seem complicated at first, but with a little practice, you’ll be able to perform this calculation quickly and easily.
One common pain point for students learning how to calculate percentage by mass is simply understanding what it means. It can be difficult to wrap your head around the idea of a substance being a certain percentage of the total mass of a sample, but this is a core concept in chemistry that can have many practical uses in the real world.
At its core, calculating percentage by mass involves taking the mass of a particular substance and dividing it by the total mass of the sample, then multiplying that result by 100%. This will give you the percentage of the sample that is made up of that particular substance.
Some key points to remember when performing this calculation include making sure that all weights are in the same unit of measurement, and paying close attention to significant figures so that your final answer is as accurate as possible.
Calculating Percentage by Mass: A Personal Experience
When I first started learning about this calculation, I was a little intimidated by the idea of dividing and multiplying mass measurements. However, after working through some practice problems, it became much easier to understand. One trick that helped me was to make sure that I was clear on what units I was working with, and to double-check my math to avoid mistakes.
If you’re feeling nervous about this calculation, just remember that practicing is key. The more you work through problems and become familiar with the process, the easier it will become.
Common Applications of Percentage by Mass Calculations
There are many situations where you might need to know the percentage of a particular substance in a sample, such as determining the concentration of a specific element in a given compound, or figuring out the composition of a complex mixture.
For example, imagine you are studying the properties of a particular alloy, and you need to know the percentage of each element in the sample. By performing a percentage by mass calculation, you can determine the exact composition of the alloy, which can be crucial for understanding its properties and potential uses.
Step-by-Step Guide to Percentage by Mass Calculations
To perform a percentage by mass calculation, follow these steps:
- Weigh the sample to determine its total mass.
- Weigh the substance you’re interested in to find its mass.
- Divide the mass of the substance by the total mass of the sample.
- Multiply the result by 100% to get the percentage by mass.
For example, imagine you have a sample of copper sulfate that weighs 200 grams. You want to know the percentage of copper in the sample. You weigh the copper and find that it weighs 80 grams. To calculate the percentage by mass of copper in the sample, you would perform the following calculation:

Therefore, the copper in the sample makes up 40% by mass.
Common Mistakes to Avoid
When working on percentage by mass calculations, there are a few mistakes that are easy to make if you’re not careful. For example, forgetting to convert all mass measurements to the same unit can lead to an incorrect answer, as can rounding too early in the calculation process.
To avoid these pitfalls, make sure to double-check your work and pay careful attention to the units you’re working with. It’s also a good idea to use significant figures throughout the calculation to ensure that your final answer is as accurate as possible.
Frequently Asked Questions About Percentage by Mass Calculations
1. What is percentage by mass?
Percentage by mass is a calculation used in chemistry to determine the amount of a particular substance in a sample based on its weight.
2. What is the formula for percentage by mass?
The formula for percentage by mass is:

3. What are some common applications of percentage by mass calculations?
Percentage by mass calculations are used in a wide variety of applications in chemistry, such as determining the concentration of a certain element in a sample, or figuring out the composition of a complex mixture.
4. What are some common mistakes to avoid when performing percentage by mass calculations?
Some common errors to watch out for when working on percentage by mass calculations include forgetting to convert units, rounding too early in the calculation process, and making errors in the math itself. To avoid these mistakes, be sure to carefully double-check your work and use significant figures throughout the calculation.
Conclusion of How to Calculate Percentage by Mass Chemistry
Calculating percentage by mass is a crucial skill for anyone studying chemistry, and it can have many important practical applications in the real world. By following the steps outlined in this guide and being mindful of common mistakes, you can become confident in your ability to perform this calculation accurately and efficiently.
Gallery
Chemistry – Percentage Composition-of Copper Hydroxide
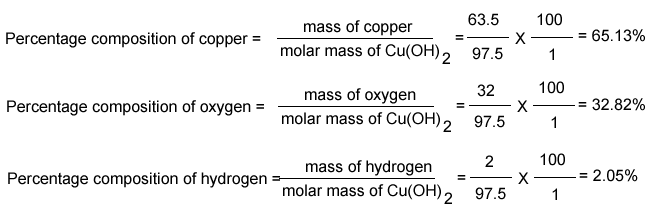
Photo Credit by: bing.com / percentage chemistry mass calculate element oh cu each composition copper hydroxide
How To Calculate Mass Percent.
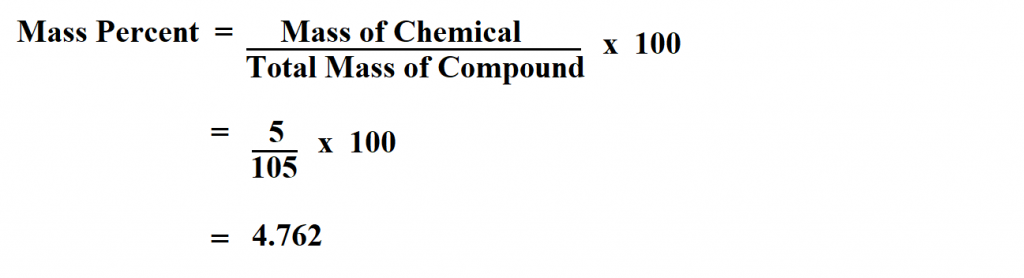
Photo Credit by: bing.com / mass naoh therefore
How To Calculate Mass Percent Of A Solution Given Density

Photo Credit by: bing.com / calculate percentage massa compound cfu calcolare percentuale molecular residual molare
How To Calculate Mass Percent Composition
/mass-percent-composition-example-609567_V2-01-89c18a9d30ea43b494d09b81f7ffefc1.png)
Photo Credit by: bing.com / mass percent composition example calculate fill
How To Find Percentage Above A Number – Howto
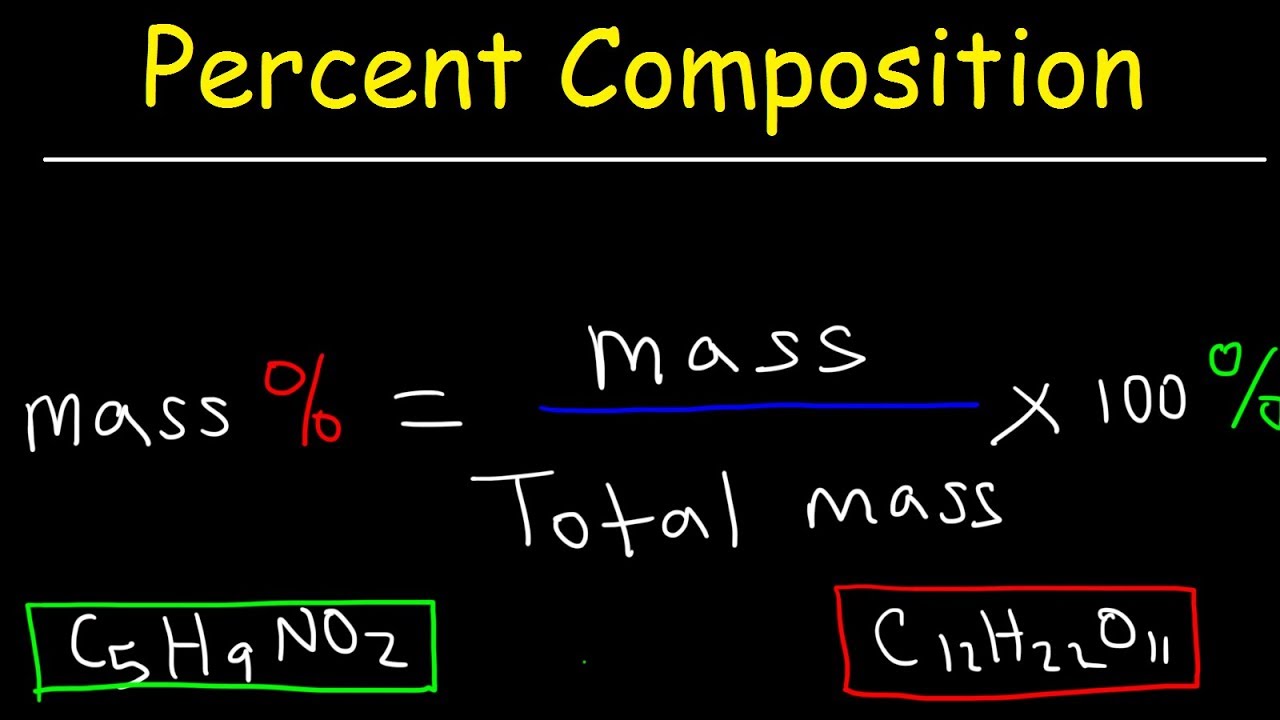
Photo Credit by: bing.com / percentage equations balance