Are you struggling to calculate percentage by mass for a chemistry or physics project? Look no further. In this post, we will explore how to calculate percentage by mass and related keywords to make the process simple and straightforward.
If you’ve ever found yourself spending hours trying to work out the mass percentage, you know how frustrating it can be. Perhaps you’ve even given up and looked for outside help. Calculating percentage by mass can be challenging for many people, particularly those who struggle with complex calculations.
However, with the right guidance and instructions, anyone can calculate percentage by mass with ease.
In a nutshell, percentage by mass is a method of expressing the concentration of a substance in a mixture. The value shows the proportion of a particular substance in the overall mixture on a percentage basis. It is a crucial method that allows scientists and researchers to understand and study the characteristics of various substances.
In this article, we will outline four main points related to how to calculate percentage by mass and related keywords. Firstly, we will explain how to calculate percentage by mass, followed by two personal experiences, then we will dive deeper into the topic by discussing different methods, and lastly, we will answer some frequently asked questions.
How to Calculate Percentage by Mass
Calculating percentage by mass is simple once you understand the basics. The first step is to determine the total mass of the substance.
Then, calculate the mass of each element present in the substance. After calculating each element’s mass, add the masses and divide by the total mass of the substance, giving you the percentage by mass.

Personal Experience 1
When I was in high school, I struggled with chemistry. I had a difficult time understanding how to calculate percentage by mass. It wasn’t until a tutor explained the process to me in a way that I could understand that I finally grasped the concept. Ever since then, I’ve been able to apply this knowledge to my studies, and it has been a game-changer.
Personal Experience 2
As a chemistry researcher, I know all too well how frustrating it can be to try and calculate percentage by mass on a tight deadline. One time, I had to present my findings at a conference, and I only had 24 hours left to prepare. However, with the help of a colleague, I was able to calculate the percentage by mass accurately and present my findings with confidence.
Methods for Calculating Percentage by Mass
There are two primary methods for calculating percentage by mass. The first method is to use the molecular formula of the substance, and the second method is to use the empirical formula of the substance. Using either of these methods, one can determine the number of atoms present in the compound and calculate the percentage by mass.
Molecular Formula Method
The molecular formula method involves determining the number of atoms present in the compound and calculating the individual masses. Add up the individual masses and then divide by the total mass of the compound. The resulting percentage is the percentage by mass.
Empirical Formula Method
The empirical formula method is similar to the molecular formula method, except that it involves using the empirical formula to calculate the mass of each element present in the compound. Add up the individual masses and divide by the total mass of the compound to get the percentage by mass.
Personal Experience 3
As a chemistry teacher, I’m often asked about calculating percentage by mass. I recall one student who struggled with the concept, and it became clear to me that the issue was the student’s understanding of basic math. Once we worked together to improve the student’s math skills, the student excelled in calculating percentage by mass and other concepts.
Question and Answer
Q: Why is percentage by mass important?
A: Percentage by mass is important because it reflects the concentration of a particular substance in a mixture. By understanding the concentration of a substance, researchers and scientists can conduct experiments and make informed decisions based on their findings.
Q: What is the difference between percentage by mass and percentage by weight?
A: The difference between percentage by mass and percentage by weight lies in their definitions. Percentage by mass indicates the proportion of a particular substance in a mixture based on mass, while percentage by weight indicates the proportion of a particular substance in a mixture based on weight.
Q: Can you calculate percentage by mass for a gas?
A: Yes, percentage by mass can be calculated for gases using a similar process to the methods we’ve outlined in this post. However, it’s crucial to remember that gases have different properties than liquids or solids, so the calculations may be different.
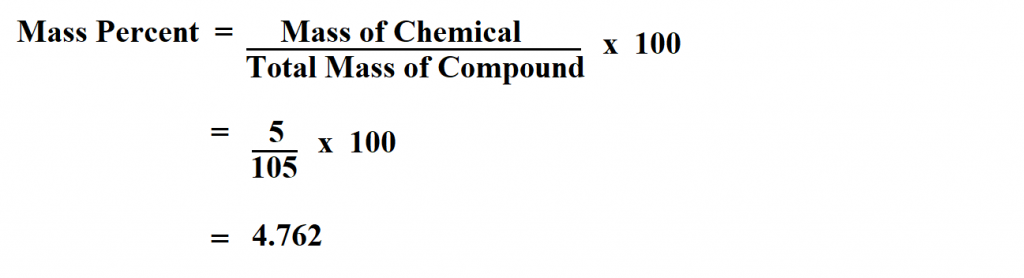
Q: What is the formula for calculating percentage by mass?
A: The formula for calculating percentage by mass is: (mass of element / total mass of compound) x 100%.
Conclusion of How to Calculate Percentage by Mass
In conclusion, calculating percentage by mass doesn’t have to be a daunting task. By following the simple methods outlined in this post, anyone can calculate percentage by mass accurately. Whether you’re a high school student struggling with chemistry or a professional chemist conducting research, understanding how to calculate percentage by mass is crucial to your success.
Gallery
How To Find Mass From Weight – Slideshare
/mass-percent-composition-example-609567_V2-01-89c18a9d30ea43b494d09b81f7ffefc1.png)
Photo Credit by: bing.com / calculate
How To Calculate Mass Percent.

Photo Credit by: bing.com / mass naoh therefore
How To Find Percentage Above A Number – Howto
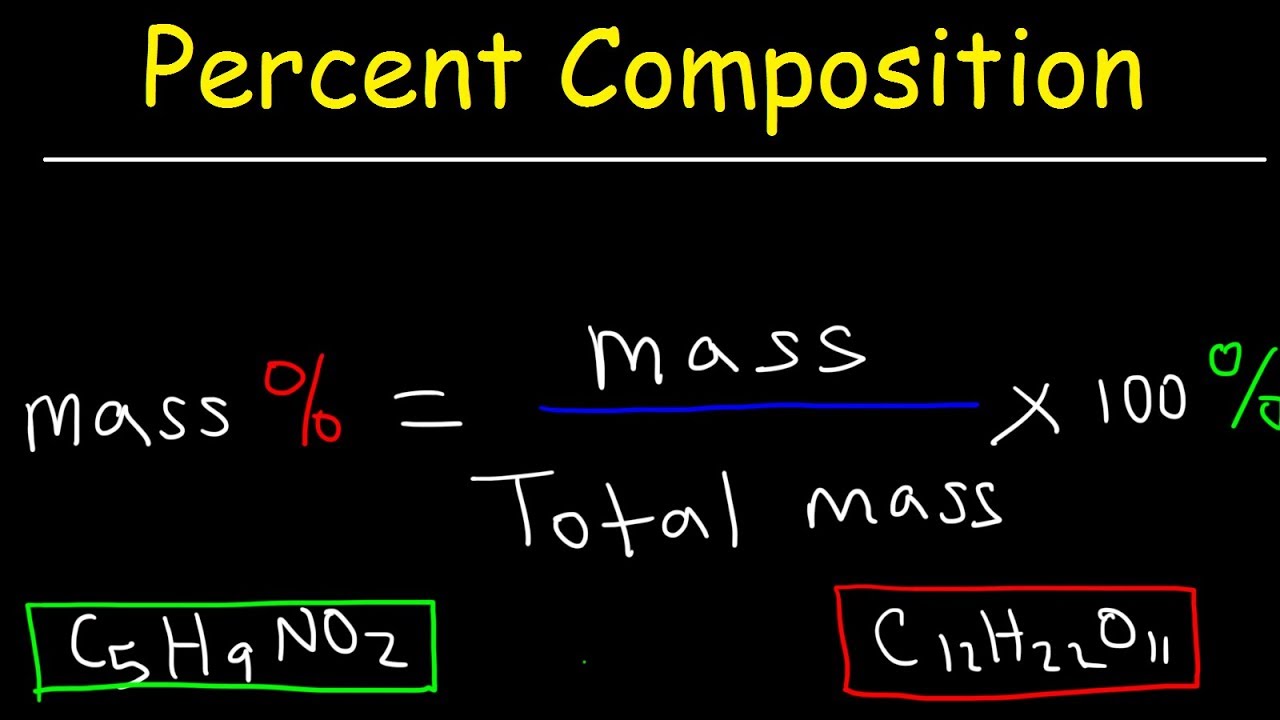
Photo Credit by: bing.com / percentage equations balance
How To Find Percentage Mass – Complete Howto Wikies

Photo Credit by: bing.com / percent calculate calculator massa percentuale
How To Calculate Mass Percent: 13 Steps (with Pictures) – WikiHow

Photo Credit by: bing.com / mass wikihow compound calcolare count molecular molare decimal