If you’re reading this, chances are you’re struggling with how to calculate percentage by mass of oxygen. Fear not! This process may seem daunting at first, but with a little guidance, you’ll be a pro in no time.
When it comes to calculating the percentage by mass of oxygen, many people get stuck on the math involved. Others may not know what values to plug in or how to use the formulas available. Regardless of where you get stuck, it’s important to approach the process with patience and a willingness to learn.
To calculate the percentage by mass of oxygen, you’ll need to know the mass of oxygen present in the compound and the total mass of the compound. Once you have those values, plug them into the following formula:
Percentage by mass of oxygen = (Mass of oxygen / Total mass of compound) x 100%
It may be helpful to use a calculator or spreadsheet to ensure accurate calculations.
In summary, to calculate the percentage by mass of oxygen, you need to first determine the mass of oxygen present in the compound and the total mass of the compound. Then plug those values into the formula and calculate accordingly. By following these steps, you’ll be able to determine the percentage by mass of oxygen with ease.
Understanding the Formula for Calculating Percentage by Mass of Oxygen
When it comes to understanding the formula for calculating the percentage by mass of oxygen, a personal experience may be helpful.
Back in high school chemistry class, I struggled with this formula for quite some time. I had trouble determining which values to plug in and how to properly calculate using the formula. However, after seeking extra help from my teacher and practicing with various compounds, I was able to finally grasp the concept.
Essentially, the formula is saying that the percentage of the mass of oxygen in a compound is equal to the mass of oxygen divided by the total mass of the compound, multiplied by 100%. By multiplying the result by 100, you convert the decimal value to a percentage.
It’s important to note that this formula only works if you know the mass of oxygen and the total mass of the compound. If you’re missing either of these values, you’ll need to go back and determine them before proceeding with the calculation.
Common Mistakes to Avoid When Calculating Percentage by Mass of Oxygen
One common mistake people make when calculating the percentage by mass of oxygen is forgetting to include all of the oxygen present in the compound. It’s important to take into account all instances of oxygen, even if they’re not in the form of an O2 molecule.
Another mistake is incorrectly determining the total mass of the compound. This value includes all atoms present in the compound, not just the oxygen. Be sure to add up the masses of all atoms in the compound to obtain the total mass.
Finding the Mass Percentage of Oxygen in a Sample
When it comes to finding the mass percentage of oxygen in a sample, it’s important to note that the process is similar to finding the percentage by mass of oxygen. However, in this case, you’ll need to know the mass of the sample and the mass of the oxygen in the sample.
To find the mass percentage of oxygen, plug the mass of oxygen and the mass of the sample into the following formula:
Mass percentage of oxygen = (Mass of oxygen / Mass of sample) x 100%
Practice Makes Perfect
As with any new concept, it may take some practice to fully grasp how to calculate the percentage by mass of oxygen. However, by following the formula and double-checking your calculations, you’ll soon be able to do so with ease.
Question and Answer
Q: What is the significance of calculating the percentage by mass of oxygen?
A: The percentage by mass of oxygen can be helpful in analyzing the properties and behavior of a given compound. It allows you to determine the relative amount of oxygen present, which can provide insights into how the compound reacts with other substances.
Q: Can this formula be used to calculate the percentage by mass of other elements in a compound?
A: Yes, the same formula can be used to find the percentage by mass of any element in a compound. Simply substitute the mass of the desired element for the mass of oxygen in the formula.
Q: How do I know if my calculations are correct?
A: Double-check your calculations and be sure to use accurate values for the mass of oxygen and the total mass of the compound. If you’re still unsure, ask a teacher or tutor for guidance.
Q: What are some common compounds that can be analyzed using this formula?
A: Examples include carbon dioxide (CO2), water (H2O), and methane (CH4).
Conclusion of How to Calculate Percentage by Mass of Oxygen
Calculating the percentage by mass of oxygen may seem difficult at first, but with practice and patience, it’s a concept that can be easily mastered. By understanding the formula, avoiding common mistakes, and double-checking your calculations, you’ll be able to accurately determine the percentage by mass of oxygen in any given compound.
Gallery
Molar Mass Of Oxygen In G/mol And Kg/mol – Ventuneac

Photo Credit by: bing.com / molar mol cloudshareinfo molecule
What Is The Mass Of Percent Of Oxygen In The Compound `UO_(2)(C_(2)H_(3

Photo Credit by: bing.com /
Oxygen On Earth | Math Encounters Blog

Photo Credit by: bing.com / oxygen percentage atmosphere earth mass calculating figure mathscinotes
Solved: Calculate The Percentage Of Carbon By Mass In Each… | Chegg.com
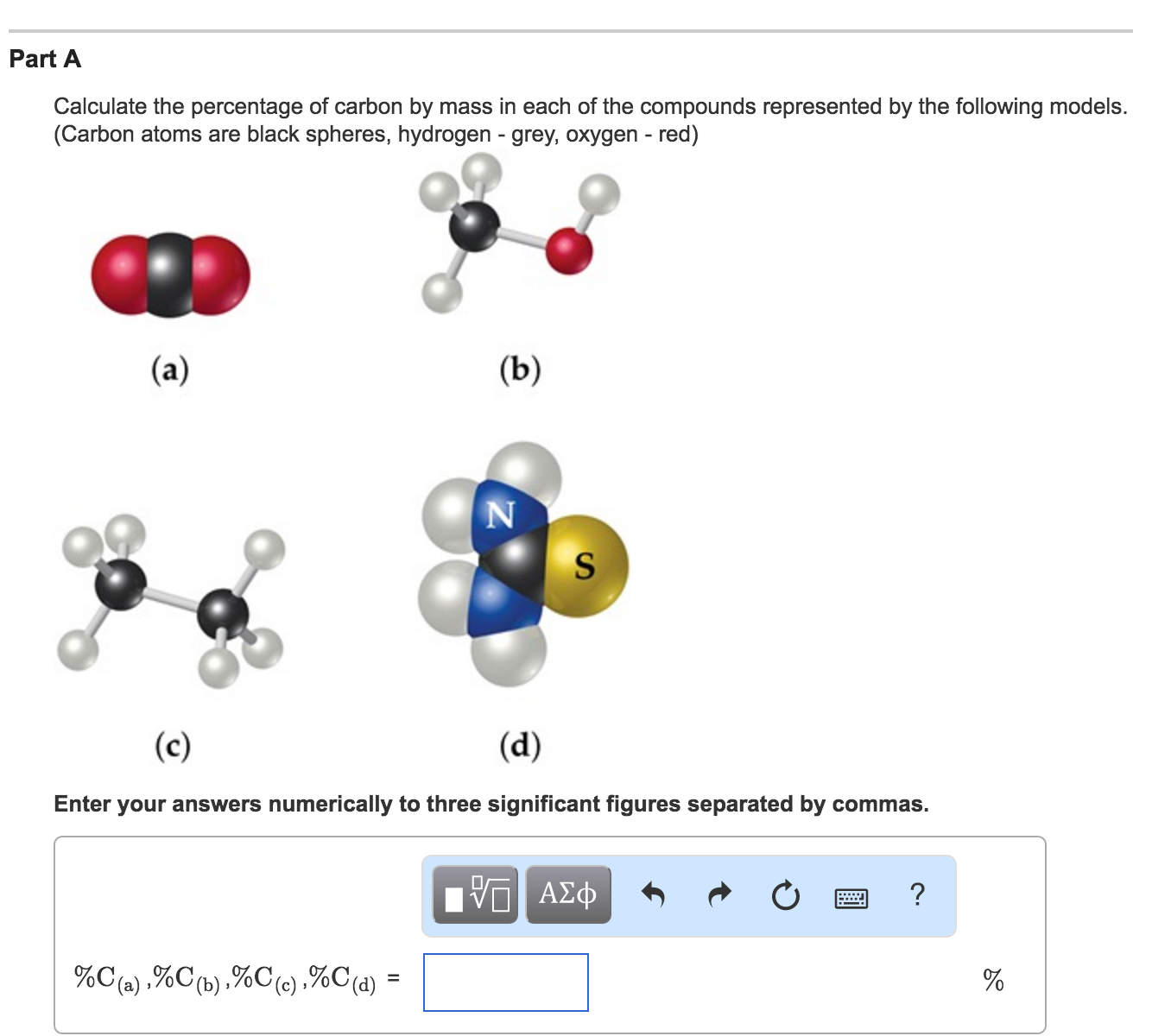
Photo Credit by: bing.com / carbon mass percentage calculate each represented following compounds models atoms oxygen red hydrogen spheres grey solved transcribed text answers
Calculate The Mass Percentage Of Oxygen Present In The Following
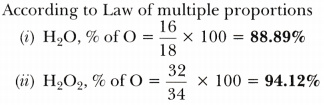
Photo Credit by: bing.com / mass water calculate present oxygen percentage compounds following combination