Have you ever wondered how to calculate percentage composition in chemistry? Are you struggling to figure out the percentage of each element in a compound? Look no further, as this post will guide you through the process of calculating percentage composition and provide useful tips along the way.
Calculating percentage composition can be a daunting task for many students. It requires a solid understanding of the molar mass of compounds and the composition of their elements. Many students struggle with balancing equations and converting between moles and grams, making the process of calculating percentage composition even more challenging.
First, let’s define percentage composition. Percentage composition is the percentage by mass of each element in a compound. In simple terms, it tells us how much of a compound is made up of each element.
To calculate percentage composition, we need to know the molar mass of the compound and the molar mass of each element in the compound. Once we have these values, we can calculate the percentage of each element in the compound using the following formula:
Formula for Calculating Percentage Composition
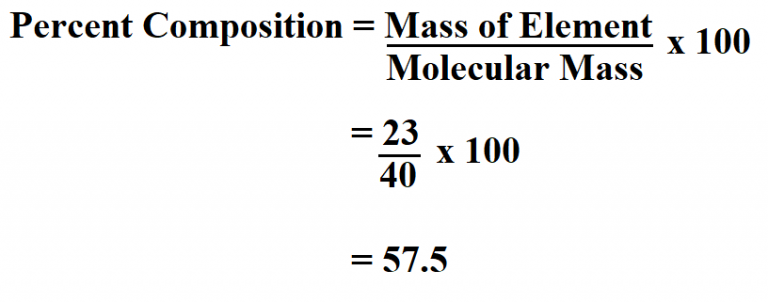
Where:
- mass of element = the mass of the element being evaluated
- mass of compound = the total mass of the entire compound
Let’s take a closer look at an example to better understand how to calculate percentage composition.
Example of Calculating Percentage Composition
/mass-percent-composition-example-609567_V2-01-89c18a9d30ea43b494d09b81f7ffefc1.png)
Suppose we want to calculate the percentage composition of sodium chloride (NaCl). We know that the molar mass of NaCl is 58.44 g/mol, and the molar mass of sodium (Na) is 22.99 g/mol, and the molar mass of chlorine (Cl) is 35.45 g/mol.
To calculate the percentage composition, we first need to determine the mass of each element in the compound. To do this, we can use the following formula:

We can then plug in the values for NaCl and solve for mass of Na and Cl:

Now that we know the mass of each element in the compound, we can use the formula for calculating percentage composition to determine the percent composition of Na and Cl in NaCl:
/mass-percent-composition-example-609567_V2-01-89c18a9d30ea43b494d09b81f7ffefc1.png)
Therefore, the percentage composition of NaCl is 39.34% sodium and 60.66% chlorine.
Tips for Calculating Percentage Composition
Calculating percentage composition can be a challenging task, but there are a few tips that can make the process easier:
- Make sure all values are in the correct units (grams and moles)
- Round final answers to the nearest tenth percent
- Double-check calculations for accuracy
- If given a compound with a hydrate, subtract the mass of the water to get the actual mass of the compound
FAQs About Calculating Percentage Composition
Q: What is the difference between empirical and molecular formulas?
A: The empirical formula is the simplest whole number ratio of the elements in a compound, while the molecular formula gives the exact number of each element in a compound.
Q: Can percentage composition be used to identify an unknown compound?
A: Yes, percentage composition can be used in conjunction with other tests to identify unknown compounds.
Q: Why is it important to know percentage composition?
A: Percentage composition is important because it helps us understand the properties and behavior of compounds. It is also useful in chemical analysis and identifying unknown substances.
Q: Can percentage composition be calculated for gases?
A: No, percentage composition is typically only calculated for solid and liquid compounds.
Conclusion
Calculating percentage composition is an essential skill in chemistry. By understanding the molar mass of a compound and the mass of each element in the compound, we can determine the percentage of each element in the compound. Although it can be a challenging task, with practice and attention to detail, you can master the art of calculating percentage composition.
Gallery
Percent Composition, Empirical And Molecular Formulas – Presentation
Photo Credit by: bing.com / percentage composition find percent empirical molecular calculating formulas formula chemistry given
How To Calculate Percent Composition.
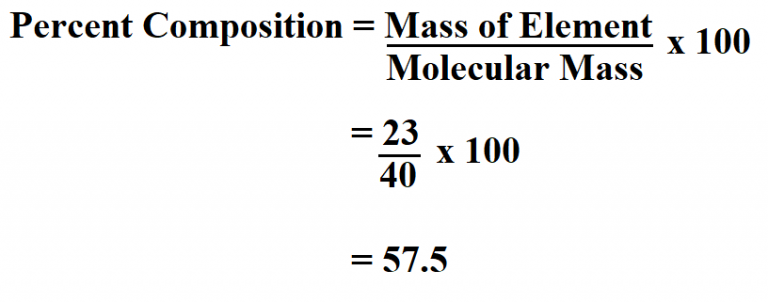
Photo Credit by: bing.com / percent calculate sodium thus
Percent Composition
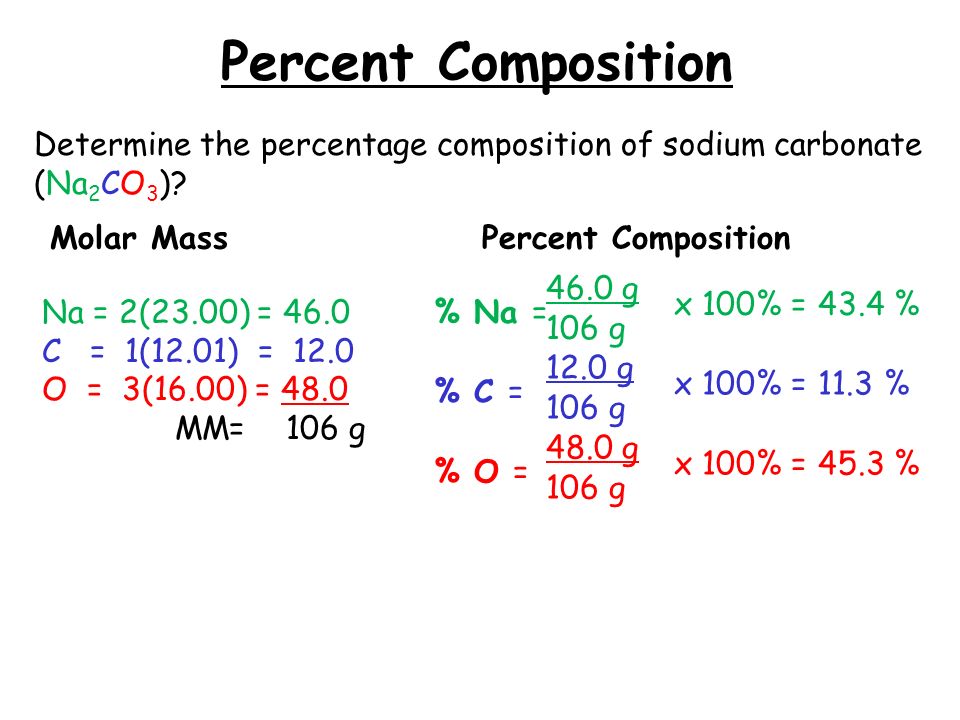
Photo Credit by: bing.com / composition percent percentage mass calculating determine try these sodium
PERCENT COMPOSITION
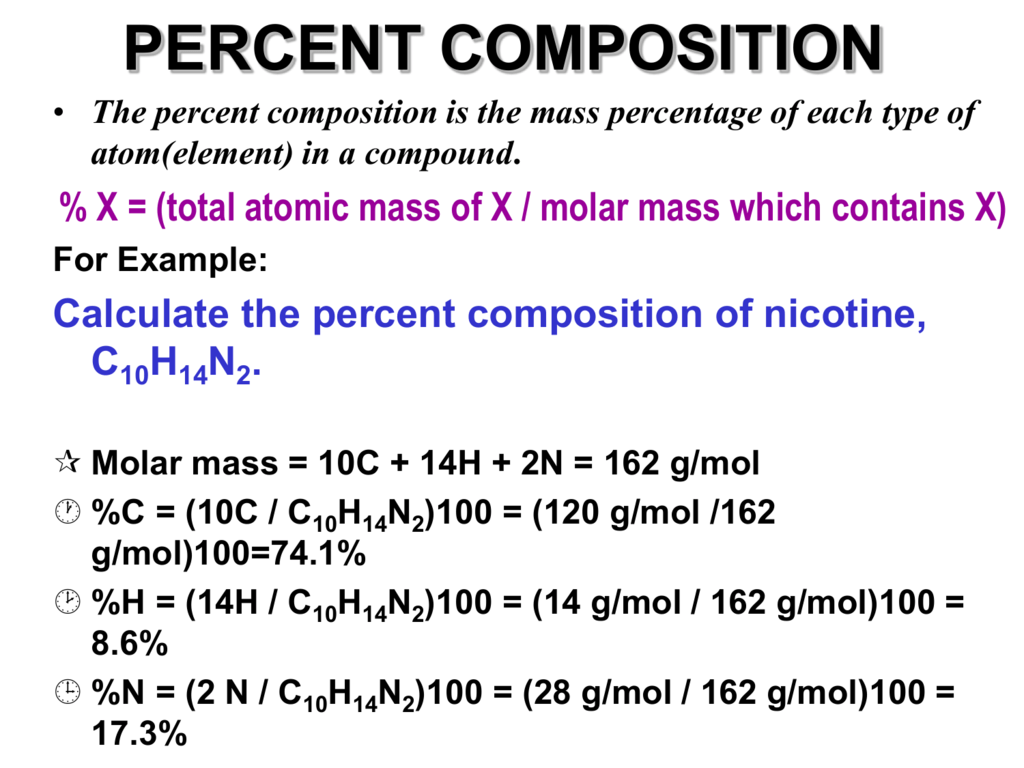
Photo Credit by: bing.com / composition percent mass percentage compound presentation atomic powerpoint slideserve element each atom ppt
How To Calculate Mass Percent Composition
/mass-percent-composition-example-609567_V2-01-89c18a9d30ea43b494d09b81f7ffefc1.png)
Photo Credit by: bing.com / mass percent composition example calculate fill
.PNG)