If you’re interested in chemistry, you may have heard the term “percentage concentration” thrown around. But what exactly does it mean, and how do you calculate it? In this blog post, we’ll dive into the details of how to calculate percentage concentration and related keywords, so you can gain a better understanding of this important concept.
When it comes to calculating percentage concentration, it can be confusing to figure out where to start. Perhaps you’re not sure what formula to use or are having trouble wrapping your head around the concept. Whatever the case may be, we understand there can be pain points associated with learning how to calculate percentage concentration.
In short, percentage concentration refers to the amount of a substance present in a solution. It is typically represented as a percentage, and can be calculated using a few different formulas depending on the specifics of your solution.
So, let’s get into the nitty gritty of how to calculate percentage concentration and related keywords. First, it’s important to keep in mind that there are a few different methods for calculating percentage concentration, including mass/volume, volume/volume, and mass/mass. Each method requires a slightly different formula, but all involve dividing the amount of solute by the total volume (or mass) of the solution, and then multiplying by 100 to get the percentage.
Personal Experience with Percentage Concentration
During my time studying chemistry, I often struggled with understanding the concept of percentage concentration. I would get stuck on the formula and become frustrated when I couldn’t quite grasp how to apply it to my specific problem. However, after spending some time practicing and seeking out extra help from my professor, I finally began to understand the ins and outs of how to calculate percentage concentration. It was a big “aha!” moment for me.
The Mass/Volume Method
One common method for calculating percentage concentration is the mass/volume method. To use this method, you’ll need to know the mass (in grams) of the solute and the volume (in milliliters) of the solution. The formula looks like this:
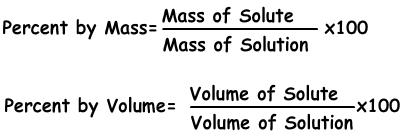
Let’s say you have 10 grams of sugar dissolved in 50 milliliters of water. To calculate the percentage concentration of the sugar, you’ll divide 10 by 50 (0.2), multiply by 100, and get 20%. So, the sugar solution is 20% concentrated.
The Volume/Volume Method
Another method for calculating percentage concentration is the volume/volume method, which is commonly used when dealing with liquid solutions. To use this method, you’ll need to know the volume of the solute and the total volume of the solution. The formula looks like this:
Percent concentration = (volume of solute / total volume of solution) x 100%
For example, let’s say you have 5 milliliters of alcohol dissolved in 30 milliliters of water. To calculate the percentage concentration of the alcohol, you’ll divide 5 by 30 (0.1667), multiply by 100, and get about 16.67%. So, the alcohol solution is 16.67% concentrated.
The Mass/Mass Method
Finally, the mass/mass method is another option for calculating percentage concentration. This method is typically used when dealing with solids. To use this method, you’ll need to know the masses of both the solute and the solution. The formula looks like this:
Percent concentration = (mass of solute / mass of solution) x 100%
For example, let’s say you have 2 grams of salt dissolved in 8 grams of water. To calculate the percentage concentration of the salt, you’ll divide 2 by 10 (the total mass of the solution), multiply by 100, and get 20%. So, the salt solution is 20% concentrated.
Question and Answer Section
Q: What is percentage concentration?
A: Percentage concentration refers to the amount of a substance present in a solution, typically represented as a percentage.
Q: What are the different methods for calculating percentage concentration?
A: The different methods for calculating percentage concentration include mass/volume, volume/volume, and mass/mass.
Q: How do I know which method to use?
A: The method you use depends on the specifics of your solution. For example, the mass/mass method is typically used when dealing with solids, while the volume/volume method is commonly used for liquid solutions.
Q: What is the formula for calculating percentage concentration?
A: The formula depends on the method you’re using. However, all methods involve dividing the amount of solute by the total volume (or mass) of the solution, and then multiplying by 100.
Conclusion of How to Calculate Percentage Concentration
Calculating percentage concentration can be a tricky concept to wrap your head around at first, but with some practice and understanding of the different methods involved, you’ll be a pro in no time. By using formulas specific to the type of solution you’re working with, you can easily calculate the percentage concentration and get a better understanding of the composition of your solution.
Gallery
Solutions Cheat Sheet | Online Chemistry Tutorials
Photo Credit by: bing.com / concentration solutions examples sheet cheat formula solution formulas percent chemistry solute solvent calculations properties
Percent Concentration Calculation (Part-04 Final) – Mass/Volume (W/V

Photo Credit by: bing.com / concentration percent volume mass calculation hindi final
Percentage Concentration Calculations – YouTube

Photo Credit by: bing.com / concentration percentage calculations
Percent Concentration Calculations With Easy Trick And Example – Part

Photo Credit by: bing.com / concentration percent calculations
Percent Concentration Calculation (Part-03) Volume By Volume (V/V) With

Photo Credit by: bing.com / volume concentration percent examples calculation