Have you ever struggled with understanding how to calculate percentage dissociation? If so, you’re not alone. Many students find this topic difficult to grasp, but fear not! In this article, we’ll walk through how to calculate percentage dissociation step-by-step, so you can ace your next exam.
When it comes to calculating percentage dissociation, students often struggle with knowing where to start. Many feel overwhelmed by the formulas and equations involved, making it difficult to know what they should focus on. Additionally, there can be confusion around whether the calculation is relating to an acid or a base. These challenges can make it feel like learning how to calculate percentage dissociation is impossible, leaving you feeling discouraged and unsure of where to turn for help.
The first step in calculating percentage dissociation involves identifying the acid or base in question. From here, we will use the ionization constant or the pH of the solution to determine the degree of dissociation. The formula for calculating percentage dissociation is as follows: % Dissociation = (amount dissociated / initial concentration) x 100. With this formula in mind, let’s dive into the step-by-step process for calculating percentage dissociation.
To calculate percentage dissociation:
Step 1: Identify the Acid or Base
It’s important to know whether the equation pertains to an acid or a base, as the process for calculating percentage dissociation will differ between the two. Once you’ve identified the acid or base, you can move onto the next step.
Step 2: Determine the Degree of Ionization
Determine the degree of ionization by using either the ionization constant (Ka or Kb), or the pH of the solution. This will help you to calculate the amount dissociated.
Step 3: Calculate the Amount Dissociated
To calculate the amount dissociated, use the equation:
amount dissociated = initial concentration x degree of dissociation
Step 4: Calculate Percentage Dissociation
Finally, use the formula % dissociation = (amount dissociated / initial concentration) x 100 to calculate the percentage dissociation.
In summary, calculating percentage dissociation involves identifying the acid or base, determining the degree of ionization, calculating the amount dissociated, and then using the formula to determine the percentage dissociation. With this process in mind, you’re well on your way to understanding how to calculate percentage dissociation!
How to Calculate Percentage Dissociation: A Personal Experience
When I first learned how to calculate percentage dissociation, I often found myself getting lost in the formulas and specific steps involved. It wasn’t until I started to take a step back and look at the bigger picture that I was able to truly understand the concept. One thing that helped me was visualizing a real-world scenario where an acid or base was dissociating. By drawing out the components and seeing how they related to each other, it became much easier for me to understand how to calculate percentage dissociation.
Common Misunderstandings About How to Calculate Percentage Dissociation
A common misunderstanding about how to calculate percentage dissociation is that it only applies to weak acids or bases. However, it’s important to note that you can apply this calculation to any acid or base, regardless of its strength.
The Importance of Understanding Percentage Dissociation
Understanding percentage dissociation is an essential part of many areas of science, including chemistry and biology. As such, it’s important to take the time to understand the process involved in calculating it. Doing so will help you to better understand the properties of acids and bases, which can lead to a stronger foundational knowledge in many scientific fields.
Final Thoughts on How to Calculate Percentage Dissociation
Learning how to calculate percentage dissociation may seem intimidating at first, but with practice and patience, it’s a skill that can be mastered. By breaking down the process step-by-step and taking the time to truly understand each component, you’ll be able to tackle even the most complex of equations. So don’t be discouraged if it takes time to fully grasp the concept – keep pushing forward, and soon enough you’ll be a pro at calculating percentage dissociation!
Question and Answer
Q1: Does percentage dissociation only apply to weak acids and bases?
A1: No, percentage dissociation can be applied to any acid or base, regardless of its strength.
Q2: What is the formula for calculating percentage dissociation?
A2: The formula for calculating percentage dissociation is: % dissociation = (amount dissociated / initial concentration) x 100.
Q3: How does the degree of ionization factor into calculating percentage dissociation?
A3: The degree of ionization helps to determine the amount dissociated, which is then used in the formula to calculate the percentage dissociation.
Q4: Can I apply percentage dissociation to non-aqueous solutions?
A4: No, percentage dissociation can only be applied to aqueous solutions.
Conclusion of How to Calculate Percentage Dissociation
Calculating percentage dissociation may seem daunting at first, but with the right approach, it’s a skill that can be mastered. By taking the time to understand each individual step and practicing regularly, you’ll be well on your way to acing your next exam. Remember; when it comes to learning how to calculate percentage dissociation, patience and persistence are key!
Gallery
Calculating The Percent Dissociation Of An Acid And The Ka Using

Photo Credit by: bing.com / dissociation percent acid ka calculating using
SOLVED:Calculate The Percent Dissociation For A 0.22-M Solution Of
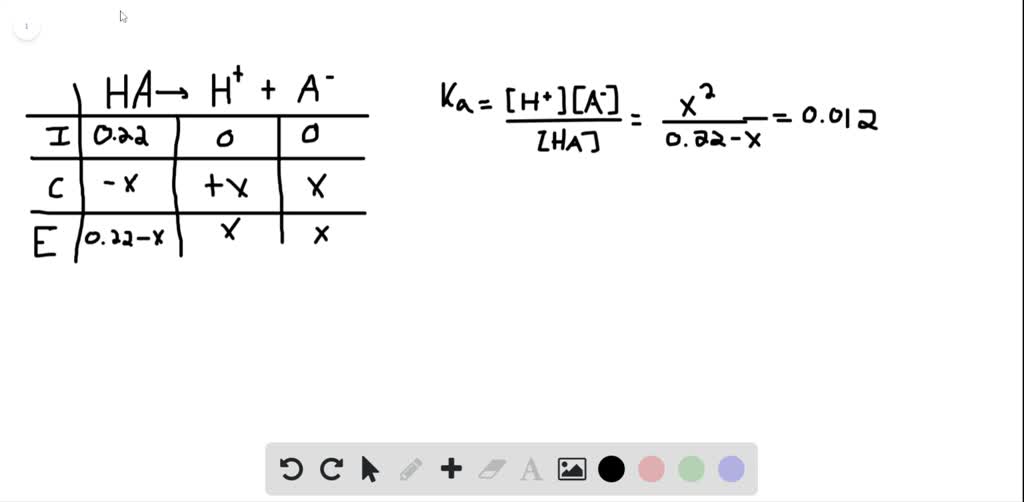
Photo Credit by: bing.com / dissociation calculate
Solved 2. Calculate The Percent Dissociation Of 0.050 M | Chegg.com
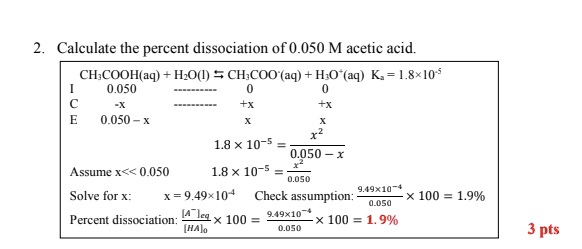
Photo Credit by: bing.com / dissociation percent calculate acid solved
Calculating Percent Dissociation – YouTube

Photo Credit by: bing.com / dissociation percent calculating
PPT – Percent Dissociation Of Weak Acids PowerPoint Presentation – ID
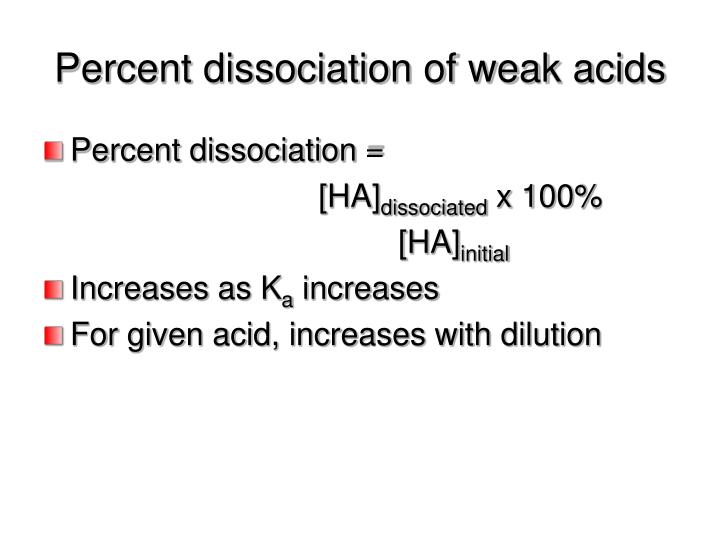
Photo Credit by: bing.com / dissociation percent weak acids presentation ppt powerpoint