Chemistry can be an intimidating subject, especially when it comes to calculating percentage mass. But understanding how to calculate percentage mass is essential for various chemical applications, including identifying unknown substances, determining chemical reactions’ products, and understanding chemical compositions.
Students and professionals alike struggle when calculating percentage mass in chemistry. It is a process that requires a thorough understanding of elemental compositions and molecular weights. Without this knowledge, it can be easy to make mistakes and produce inaccurate results.
Calculating percentage mass in chemistry involves determining the mass percentage of each element in a compound or mixture’s total mass. The percentage of each element is determined by dividing the element’s mass by the total compound or mixture’s mass and multiplying by 100.
Now that we have a basic understanding of how to calculate percentage mass in chemistry let us delve into the specifics to get a better understanding.
Understanding the Composition of a Substance
When determining the percentage mass of a substance, it is essential to understand its composition. A substance is composed of different elements, and each element within that substance has an atomic weight (mass).
To determine a substance’s percentage mass, we need to calculate the mass of each element within the compound, determine the total mass of the substance, and calculate the percentage each element contributes to the total mass.
For instance, suppose we have a hydrochloric acid solution. The solution has a mass of 100 grams, and we want to know the percentage mass of hydrogen and chlorine. We can use the formula:
mass percentage = (mass of element / total mass of the compound) x 100
The molecular formula for Hydrochloric acid is HCl. Its atomic weight is 36.5 g/mol. Therefore, one mole of HCl weighs 36.5 g.
Therefore, for 100 g of the Hydrochloric acid solution, the weight of HCl required will be: 100/36.5= 2.74 moles of HCl.
Now, we need to calculate the mass of each element. We know that Hydrogen has an atomic weight of 1 g/mol, and the compound’s only hydrogen atom is in the HCl compound, which means the mass of hydrogen in the compound is:
mass of hydrogen in the compound = 2.74 x 1 g/mol = 2.74 g of hydrogen
Next, we can calculate the mass of chlorine. The compound has only one chlorine atom, and chlorine’s atomic weight is 35.5 g/mol. Therefore,
mass of chlorine in the compound = 2.74 x 35.5g/mol= 96.8 g of chlorine.
So, the total mass of the Hydrochloric acid solution is 100 g, and the mass of hydrogen and chlorine are 2.74 g and 96.8 g, respectively.
To find the mass percentage of hydrogen and chlorine, we use the formula above:
mass percentage of hydrogen = (2.74g / 100g) x 100 = 2.74%
mass percentage of chlorine = (96.8g / 100g) x 100 = 96.8%
Therefore, the Hydrochloric acid solution has a mass percentage of hydrogen and chlorine of 2.74% and 96.8%, respectively.
Using Mole Fractions
Calculating percentage mass in chemistry can also be determined using mole fractions. Mole fraction is the ratio of the number of moles of a component to the total number of moles in a mixture.
To calculate the mole fraction of each element, we divide the number of moles of a particular element by the total number of moles present in the compound. We then multiply the result by 100 to obtain the percentage of that element in the compound.
For example, suppose we have a compound consisting of two elements, A and B. Element A has two moles, and element B has four moles. Therefore, the total number of moles is six.
The mole fraction of element A in the compound will be:
mole fraction of A = (number of moles of A / total number of moles) x 100 = (2/6) x 100 = 33.33%
The mole fraction of element B in the compound will be:
mole fraction of B = (number of moles of B / total number of moles) x 100 = (4/6) x 100 = 66.67%
The above calculations show that element A contributes 33.33% to the compound’s mass, while element B contributes 66.67%.
Limitations of Percentage Mass Calculations
Calculating percentage mass in chemistry is a vital process in determining the composition of a substance. However, the process is limited to pure substances or ideal mixtures. It may not provide accurate results for non-ideal or complex mixtures, such as alloys or polymers, where the chemical composition of each molecule may not be well defined.
Conclusion of how to calculate percentage mass in chemistry
Calculating percentage mass in chemistry can be a daunting task, but it is essential in the field of chemistry. By understanding the method of determination for percentage mass, we can identify unknown substances, determine product yields in chemical reactions, and understand the chemical compositions of solutions. The calculations involved require a thorough understanding of the compound’s composition, molecular weights, and the usage of mole fractions.
Frequently Asked Questions
Q: How do you calculate the percentage mass of an element in a compound?
To calculate the percentage mass of an element in a compound, divide the mass of the element by the total mass of the compound and multiply by 100.
Q: Why is it essential to calculate the percentage mass of elements in a compound?
Calculating the percentage mass of elements in a compound is essential to determine its composition. It is a fundamental step in identifying unknown substances, calculating product yields, and understanding the chemical composition of solutions.
Q: How do you calculate the percentage mass of a solution?
To calculate the percentage mass of a solution, measure the mass of the solute and add to the mass of the solvent. Then, divide the mass of the solute by the total mass of the solution and multiply by 100.
Q: What is the difference between mole fraction and mass fraction?
The mole fraction of a component in a mixture is the ratio of moles of that component to the total number of moles in the mixture. The mass fraction is the ratio of the mass of that component to the total mass of the mixture.
Gallery
How To Calculate Mass Percent Composition
/mass-percent-composition-example-609567_V2-01-89c18a9d30ea43b494d09b81f7ffefc1.png)
Photo Credit by: bing.com / mass percent composition example calculate fill
How To Calculate Mass Percent Of A Solution Given Density

Photo Credit by: bing.com / calculate percentage massa compound cfu calcolare percentuale molecular residual molare
How To Find Percentage Above A Number – Howto
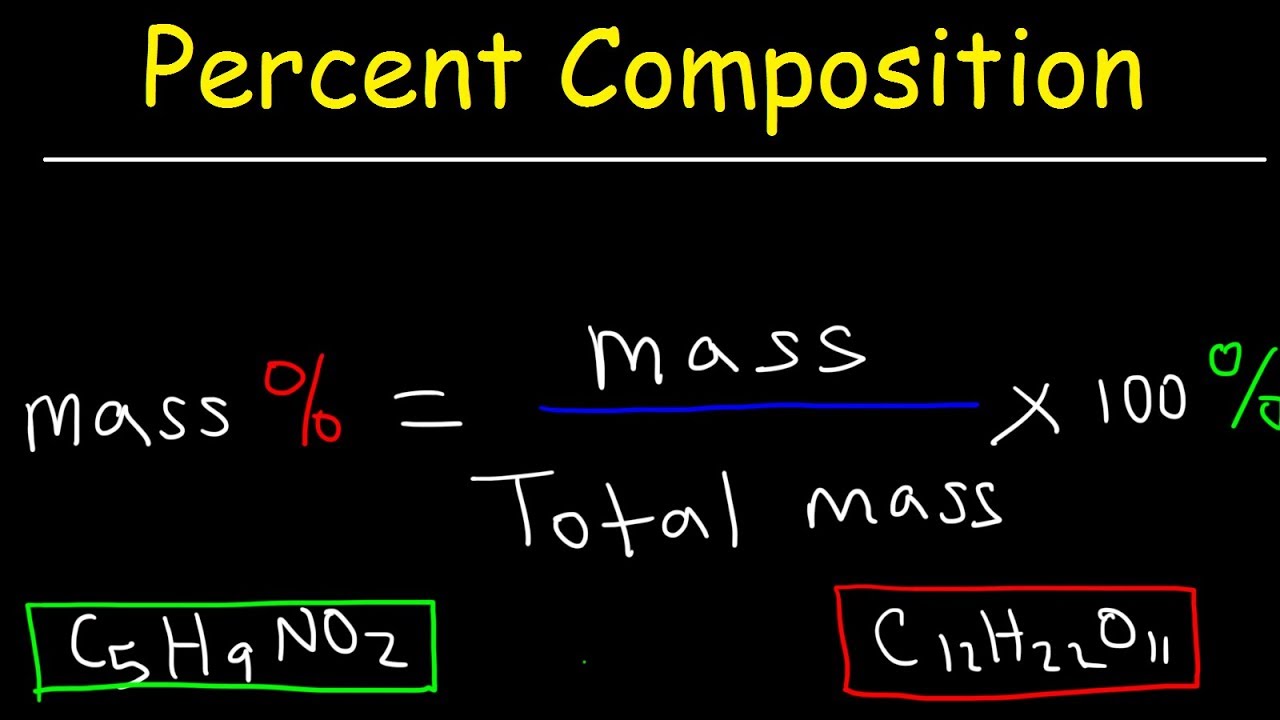
Photo Credit by: bing.com / percentage equations balance
How To Calculate Mass Percent.
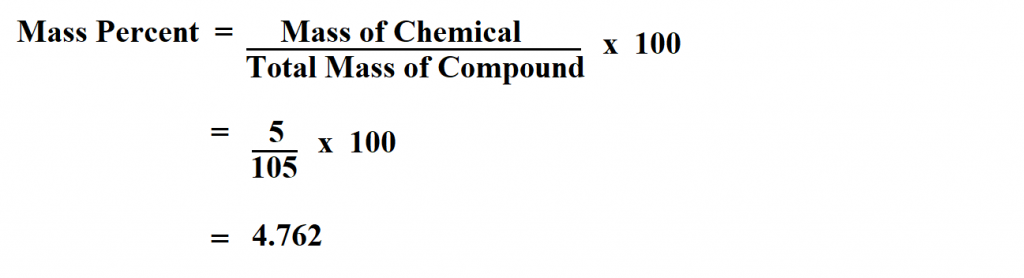
Photo Credit by: bing.com / mass naoh therefore
Chemistry – Percentage Composition-of Copper Hydroxide
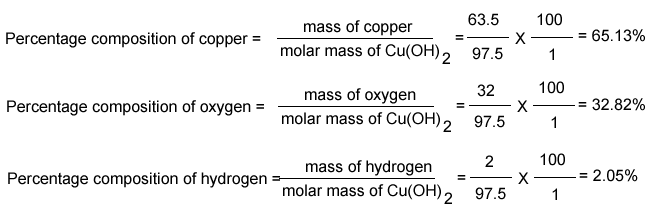
Photo Credit by: bing.com / percentage chemistry mass calculate element oh cu each composition copper hydroxide