Chemistry students often struggle with calculating the percentage purity of a substance. This vital measurement is crucial in many areas of chemistry, such as analyzing the quality of drugs or checking the purity of industrial chemicals. In this blog post, we will explore how to calculate percentage purity chemistry step by step and provide helpful tips to ensure accuracy.
Pain Points Related to How to Calculate Percentage Purity Chemistry
Although calculating percentage purity is essential, the process can be tedious, time-consuming, and confusing. The method involves a series of calculations, including weighing the sample, calculating the expected yield, and comparing the actual yield, among others. Moreover, even a slight error in measurements can result in inaccurate results, leading to misleading conclusions.
Answer: How to Calculate Percentage Purity Chemistry
Percentage purity refers to the percentage of an impure substance that is the desired pure product. The formula for percentage purity ((Actual yield) / (Expected yield) x 100) helps you find out the ratio between the two, multiplied by 100 to convert the result into a percentage.
Summary of Main Points Related to How to Calculate Percentage Purity Chemistry
Calculating percentage purity chemistry is a vital process in chemistry that helps scientists assess the quality of a substance. However, it can be tedious and confusing, with the potential to result in misleading results. The process involves several calculations, including comparing actual and expected yields and weighing the sample. Accuracy is key, and even a slight error can lead to inaccurate results.
How to Calculate Percentage Purity Chemistry: Step by Step Process
When calculating percentage purity, your aim is to determine the ratio of the actual yield to the expected yield of the desired product. Here’s a step-by-step approach:
- Weigh the sample of impure substance using an accurate scale.
- Determine the expected yield of pure product based on the balanced chemical equation.
- Perform the chemical reaction and collect the precipitate.
- Dry the precipitate to remove moisture and weigh.
- Calculate the percentage purity using the formula: Percentage purity = (Actual yield ÷ Expected yield) x 100
It is essential to use the correct balance chemical equation to determine the expected yield of the pure product. Additionally, maintaining accurate measurements and data recording is imperative when calculating percentage purity.
Common Mistakes When Calculating Percentage Purity Chemistry and How to Avoid Them
Some of the most common mistakes when calculating percentage purity include measuring the sample incorrectly, using the wrong balance equation, or not considering moisture in the precipitate. You can avoid these mistakes by using a calibrated scale to weigh samples accurately, double-checking the balance equation, and drying the precipitate thoroughly before calculations.
Tips for Calculating Percentage Purity Chemistry More Accurately
To calculate percentage purity in chemistry accurately, here are some helpful tips:

- Use calibrated glassware and a high-precision electronic balance.
- Keep accurate records and notes of every measurement.
- Ensure that the sample is homogenous and representative of the entire product.
- Avoid exposure to moisture, air, light, or temperature changes, as they can affect measurement accuracy.
- Double-check the balance equation and ensure that you have the correct stoichiometry ratios.
Question and Answer About How to Calculate Percentage Purity Chemistry
Q: What is the significance of calculating percentage purity in chemistry?
A: Calculating percentage purity is vital to ensuring the quality of substances used in many scientific fields, such as pharmaceuticals, textiles, and food industry, among others. It helps researchers and professionals determine the percentage of the desired product in a sample, assess the purity of products or raw materials, and ensure safety and efficiency standards.
Q: What are the potential sources of errors when calculating percentage purity?
A: Some potential sources of errors are incorrect measurements, improper preparation of the sample, impurities in the reagents, incorrect stoichiometry ratios, lack of calibration of the equipment, or inaccurate data recording.
Q: How can I interpret the results of percentage purity calculations?
A: The higher the percentage purity of a sample, the more likely it is to contain a predominant amount of the desired product. Conversely, a lower percentage purity indicates that the sample contains more impurities and less of the desired product.
Q: Can percentage purity have a value over 100%?
A: No, percentage purity can have a maximum value of 100%. Even if the actual yield is greater than the expected yield, the percentage purity value will still be 100%.
Conclusion of How to Calculate Percentage Purity Chemistry
Calculating the percentage purity of a substance is critical in various scientific fields that require assessing the purity and quality of substances. Precision and accuracy are essential when performing this calculation, and errors can lead to misleading results. Through proper preparation, measurements, and attention to detail, you can calculate percentage purity accurately and efficiently.
Gallery
️ How To Calculate Percentage Purity Of An Impure Sample. How To
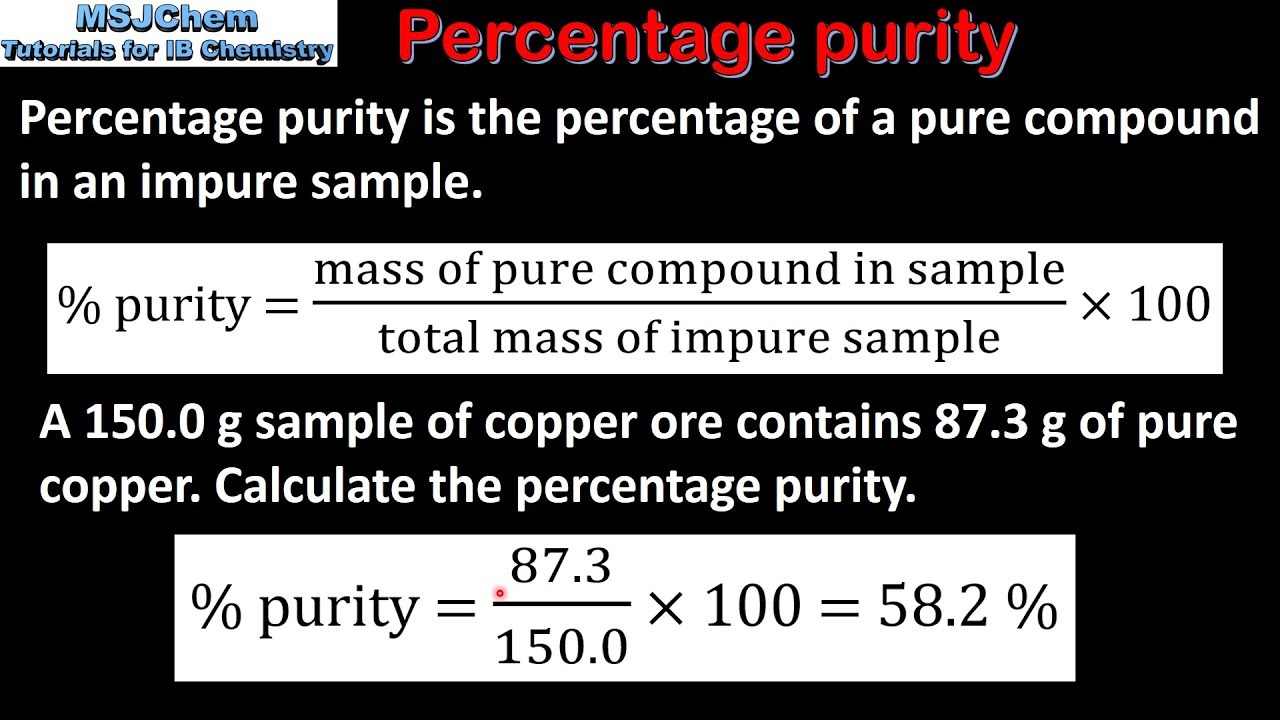
Photo Credit by: bing.com / purity percentage calculate sample impure determine compound hplc using
Question Video: Determining The Equation To Calculate Percentage Purity

Photo Credit by: bing.com /
S.L.A.M. Chem Notes: April 2011
Photo Credit by: bing.com / chem notes practice need chemistry
What Is The Formula For Percentage Purity? – Quora
Photo Credit by: bing.com / purity formula percentage example
How To Find Percentage Above A Number – Howto
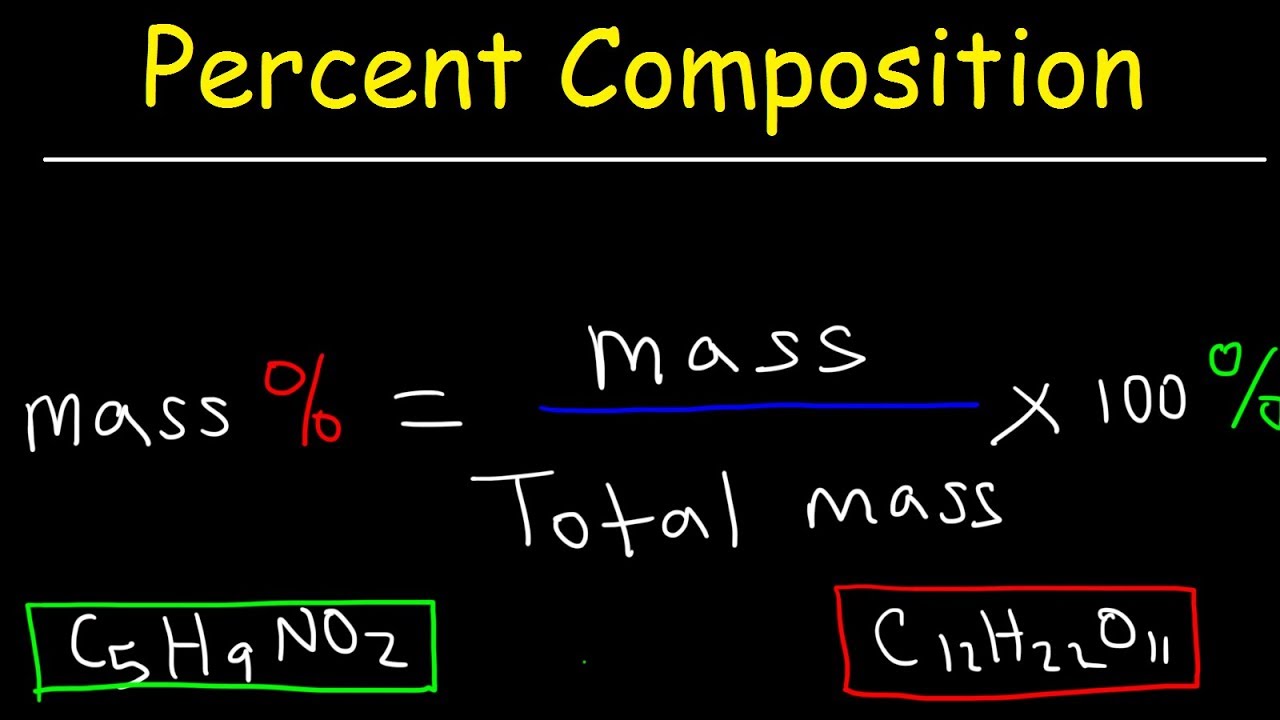
Photo Credit by: bing.com / percentage equations balance