Have you ever wondered how to calculate percentage purity? Whether you’re a chemistry student or just curious about the purity of the products you use, calculating percentage purity is a critical skill to have. Knowing how to determine the percentage of a compound that is pure can not only help you with your studies but also ensure the safety and quality of the products you use in your daily life.
When it comes to calculating percentage purity, there are a few pain points that many people face. Figuring out where to start, understanding the equation to use, and interpreting the results can all be confusing and overwhelming for beginners.
Simply put, calculating percentage purity involves determining the amount of pure compound in a sample. The formula to calculate the percentage purity of a compound is:

Where:
- Pure mass – the mass of the pure compound (in grams)
- Sample mass – the total mass of the sample (in grams)
Now that we know the formula, let’s summarize the article’s main points, which include understanding the percentage purity formula, identifying the pure and sample masses, and interpreting the results.
How to calculate percentage purity: A personal experience
When I was in college, I had to conduct several experiments that required me to determine the purity of a compound. One experiment involved analyzing the purity of caffeine in coffee beans. To do this, I had to extract the caffeine from the beans and then determine the percentage of pure caffeine in my sample.

After extracting the caffeine and obtaining my sample, I weighed the pure caffeine and sample mass using a digital scale. I then plugged these values into the percentage purity formula and calculated the purity percentage of the caffeine in the sample.
This experience helped me understand the importance of accurately determining the percentage purity of a compound and how to use the formula to do so.
Identifying the pure and sample masses
To calculate percentage purity, we need to know the pure and sample masses. The pure mass is the mass of the compound that is pure, while the sample mass is the total mass of the sample that we’re testing. Here are some tips for identifying these values:
- The pure mass is typically given in the experiment or problem that you’re working on.
- If the pure mass is not given, you can calculate it by subtracting the mass of the impurities from the total mass of the sample.
- The sample mass is the total mass of the compound and impurities that you’re testing. Be sure to measure this accurately on a digital scale.
Interpreting the results
After plugging in the values for the pure and sample masses, you’ll get a percentage value that represents the purity percentage of the compound. The higher the percentage, the more pure the compound is.
It’s important to note that no compound is 100% pure, as there are always impurities present to some extent. However, knowing the percentage purity can help us determine if the compound is suitable for our intended use and whether any further purification steps are necessary.
Other methods for determining percentage purity
While the formula mentioned above is a common way to determine percentage purity, there are other methods available as well. These methods include chromatography, spectroscopy, and x-ray diffraction.
The importance of calculating percentage purity
Understanding how to calculate the percentage purity of a compound is crucial in various fields such as chemistry, pharmaceuticals, and food science. It helps ensure the safety and quality of products and can be used to identify when further purification is necessary.
Question and Answer
Q: Can a compound be more than 100% pure?
A: No, a compound cannot be more than 100% pure. The maximum purity percentage is 100%, as this represents the total mass of the pure compound in the sample.
Q: What if the value obtained by calculating percentage purity is less than 100%?
A: If the value obtained is less than 100%, it means that there are impurities present in the sample. The lower the percentage, the more impurities there are.
Q: What is a safe purity percentage for pharmaceutical products?
A: The percentage purity requirements of pharmaceutical products vary depending on the product and the intended use. However, as a general rule, a purity percentage of at least 98% is considered safe for most applications.
Q: What is the most common method used to determine percentage purity in the food industry?
A: In the food industry, high-performance liquid chromatography (HPLC) is the most common method used to determine percentage purity.
Conclusion of how to calculate percentage purity
Calculating the percentage purity of a compound is an essential skill that is necessary in various fields. By understanding the formula, identifying the pure and sample masses, and interpreting the results, we can accurately determine the purity of a compound, ensuring its safety and quality for our intended use.
Gallery
What Is The Formula For Percentage Purity? – Quora
Photo Credit by: bing.com / purity formula percentage example
S.L.A.M. Chem Notes: April 2011
Photo Credit by: bing.com / chem notes practice need chemistry
️ How To Calculate Percentage Purity Of An Impure Sample. How To
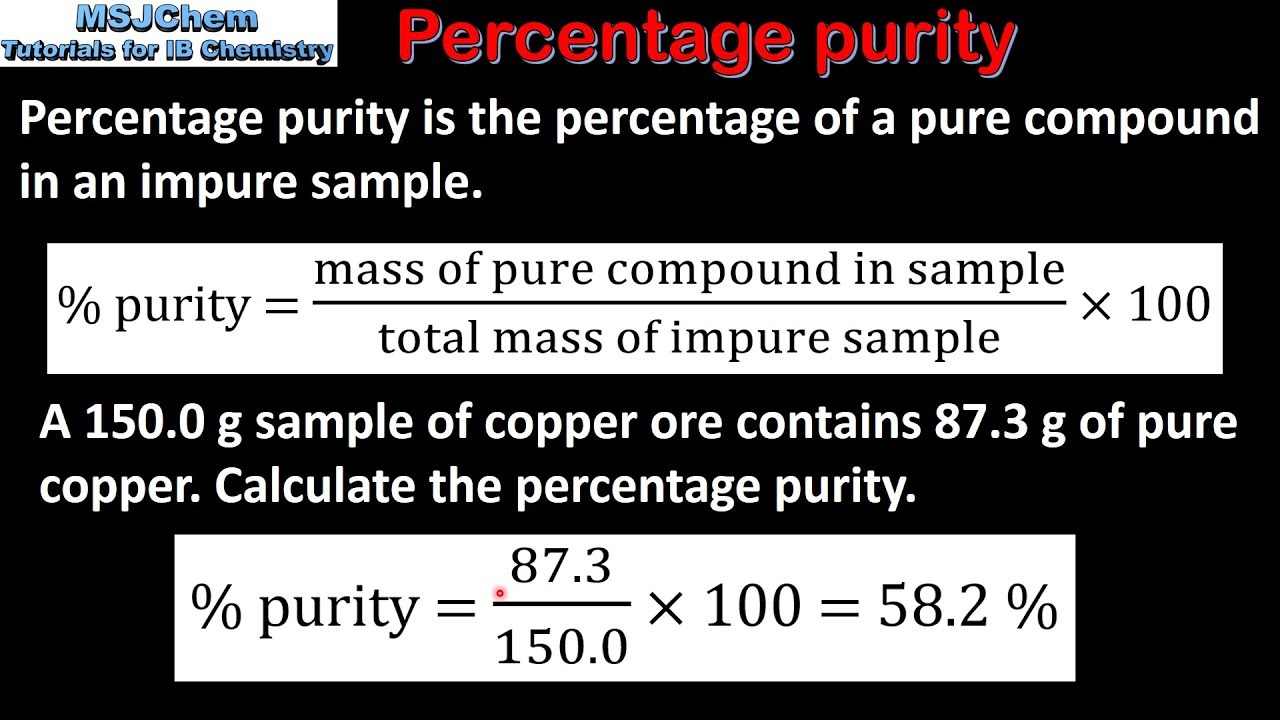
Photo Credit by: bing.com / purity percentage calculate sample impure determine compound hplc using
18 Percentage Yield
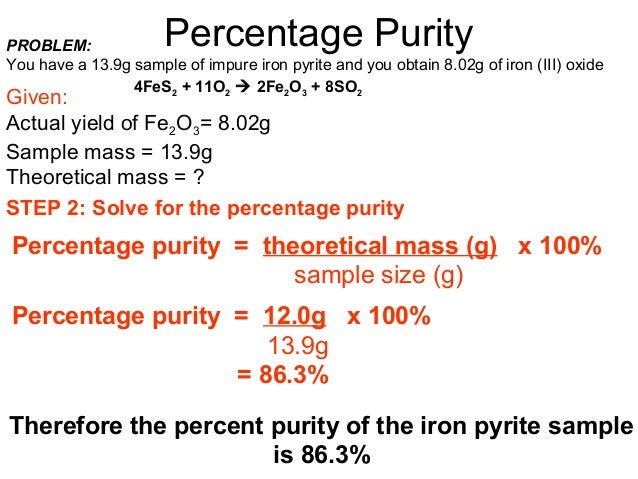
Photo Credit by: bing.com / percentage impure
Question Video: Determining The Equation To Calculate Percentage Purity

Photo Credit by: bing.com /