Have you ever wondered how to calculate percentage to ppm? Understanding this conversion can be essential in a wide range of fields, including chemistry, environmental science, and medicine. Not only is it useful for determining the concentration of a substance, but it can also help measure the quality of water, air, and other essential elements in our environment.
Pain Points Related to Calculating Percentage to PPM
Calculating percentage to ppm can be a challenging task for many individuals, especially those who are not from a scientific background. The conversion process may seem complicated, and one small mistake can significantly affect the results. Moreover, people may not know why they need to convert percentages to ppm or what it even means.
The Answer to How to Calculate Percentage to PPM
Before explaining how to calculate percentage to ppm, it is crucial to understand what ppm means. Parts per million (ppm) is a unit used to express the concentration of a certain substance in a solution or mixture. It is a measure of how many parts of a substance are present per million parts of the mixture. In other words, ppm is a ratio of the amount (in weight or volume) of the solute to the amount of solution or mixture.
To calculate percentage to ppm, you need to multiply the percentage value by 10,000. For instance, if the percentage is 5%, the calculation would be (5% x 10,000) = 50,000 ppm.
Summary of Main Points about How to Calculate Percentage to PPM
In summary, calculating percentage to ppm is a conversion process utilized in many fields. It involves multiplying the percentage value by 10,000 to get the corresponding ppm value. The process may seem complicated, but understanding it is essential to analyze the concentration of a substance in a solution or mixture, especially in environmental science, medicine, and chemistry.
Crafting Detailed Explanation on How to Calculate Percentage to PPM
I remember my first chemistry lab in high school where we had to calculate the concentration of acetic acid in vinegar. We were given the percentage value of acetic acid, and the task at hand was to convert it into ppm. I found myself struggling with the formula and calculation process, thinking that maybe chemistry was not my cup of tea. But once I understood the concept of ppm, I realized that the calculation process was not as complicated as it seemed.
To calculate ppm, you need to know the weight or volume of the solute and the weight or volume of the solution. Let’s take an example of calcium carbonate in water, where the solute (calcium carbonate) has a weight of 5 grams and is dissolved in 1000 liters of water.
First, we need to calculate the weight of the calcium carbonate in ppm, which would be (5g / 1000L) x 10^6 = 5000 ppm.
Secondly, we need to calculate the percentage of calcium carbonate, which would be (5g / 1000g) x 100% = 0.5%.
Lastly, to convert the percentage to ppm, we multiply 0.5% by 10,000, which gives us 5000 ppm.
Understanding the Importance of Calculating Percentage to PPM
Calculating percentage to ppm is essential in various fields, especially in environmental science, where it helps measure the quality of water and air. It can also help in medicine by determining the concentration of drugs in a patient’s bloodstream. Moreover, in chemistry, it is used to analyze the concentration of elements in a solution or mixture.
How to Ensure Accurate Calculations
To ensure accurate calculations, it is crucial to use the correct formula, always double-checking all the values before the calculation. Moreover, it is essential to express the units consistently throughout the calculation, including units of weight, volume, or any other relevant units.
Common Mistakes to Avoid When Calculating Percentage to PPM
One common mistake people make when calculating percentage to ppm is forgetting to convert units of weight or volume. For instance, if the solute is given in grams and the solution in milliliters, the units need to be converted for an accurate calculation.
Another common mistake is estimating or rounding off values, which can significantly affect the results. It is crucial to use exact values throughout the calculation process.
Answering Common Questions about How to Calculate Percentage to PPM
Q1. What is the difference between percentage and ppm?
Answer: Percentage is a way to express a fraction of a hundred, while ppm is a way to express a fraction of a million.
Q2. What is the formula to calculate ppm?
Answer: To calculate ppm, you need to divide the weight or volume of the solute by the weight or volume of the solution and multiply the result by 10^6.
Q3. How do you convert ppm to percentage?
Answer: To convert ppm to a percentage, you need to divide the ppm value by 10,000.
Q4. How is ppm used in environmental science?
Answer: In environmental science, ppm is used to measure the concentration of harmful substances in the air or water and ensure compliance with safety regulations.
Conclusion of How to Calculate Percentage to PPM
Understanding how to calculate percentage to ppm is an essential skill in various fields. It involves multiplying the percentage value by 10,000 to get the corresponding ppm value. Accurate calculations are crucial and require using the correct formula, ensuring consistent units, and avoiding common mistakes. By understanding the concept of ppm and its significance, we can determine the concentration of substances in different mixtures and solutions, making informed decisions about safety and quality.
Gallery
Apa How To Calculate Ppm – Lightingaceto

Photo Credit by: bing.com /
How To Calculate Ppm | Ppm Calculation – YouTube
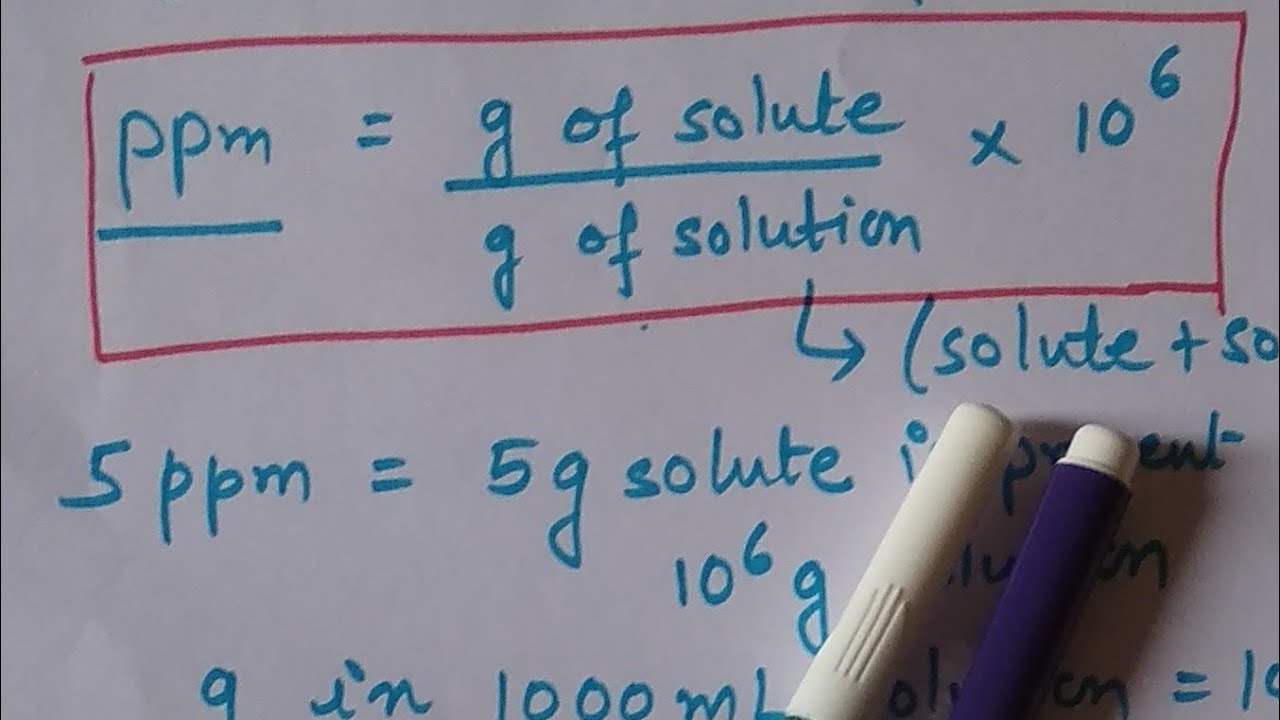
Photo Credit by: bing.com / calculation
How To Calculate Ppm – Northwestlasopa
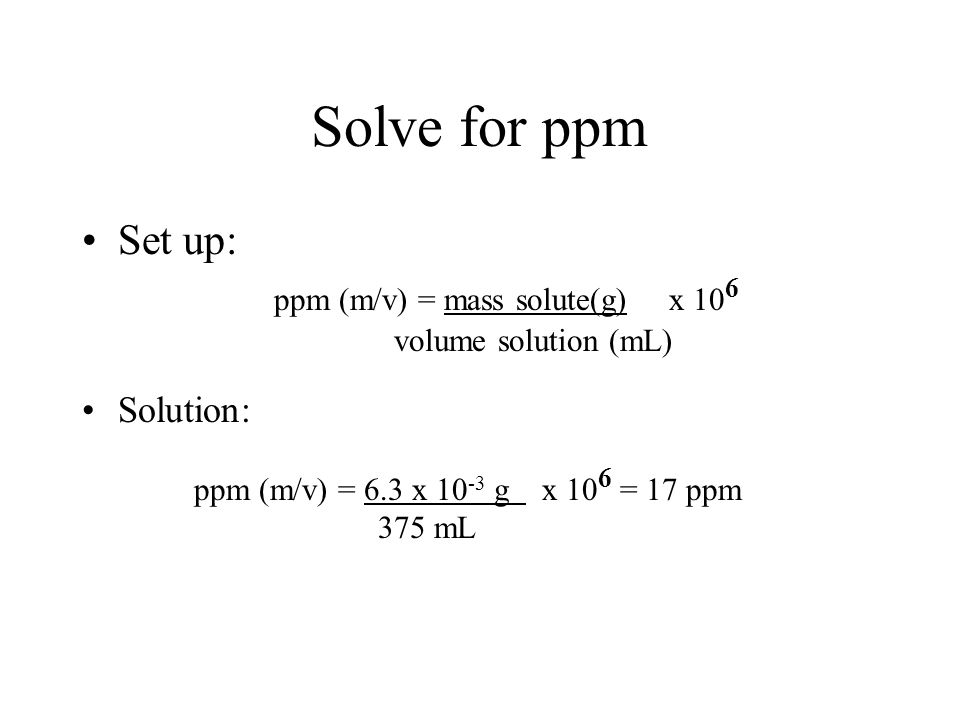
Photo Credit by: bing.com / calculate
Molarity Percent By Mass Ppm Simple Calculations – YouTube
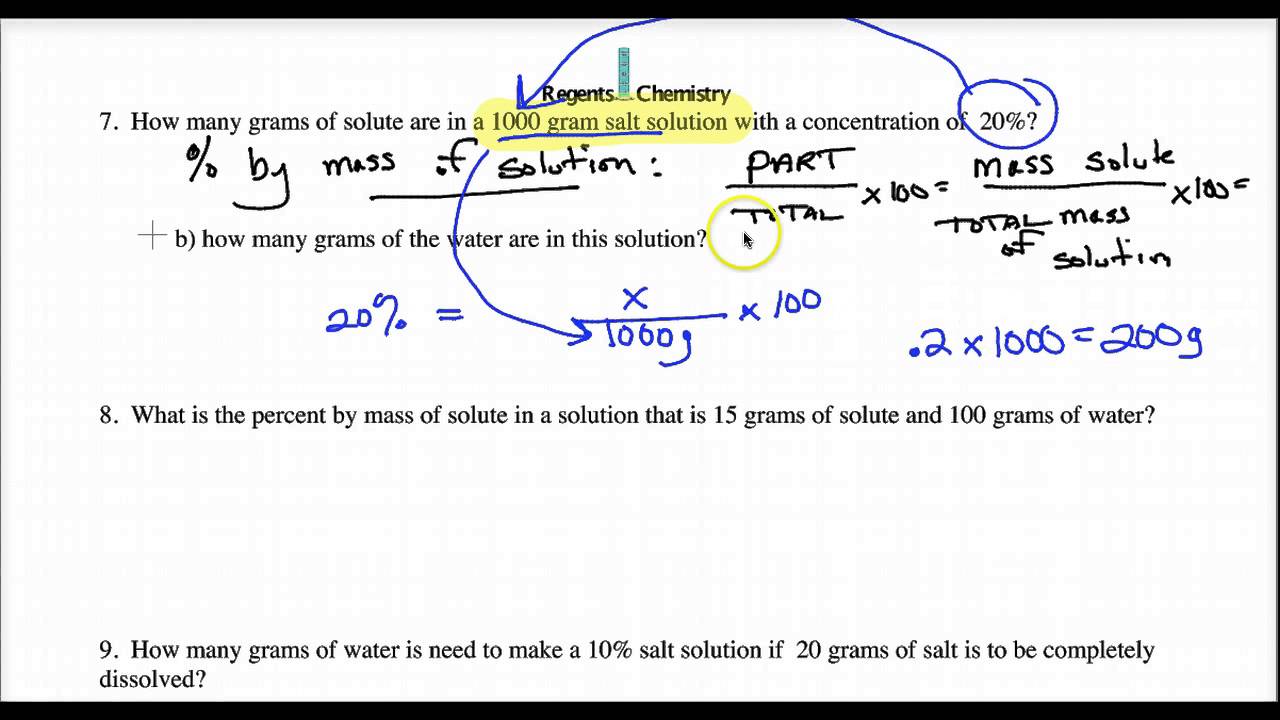
Photo Credit by: bing.com / ppm molarity percent mass calculations simple
How To Calculate Ppm In Solution – Warascse
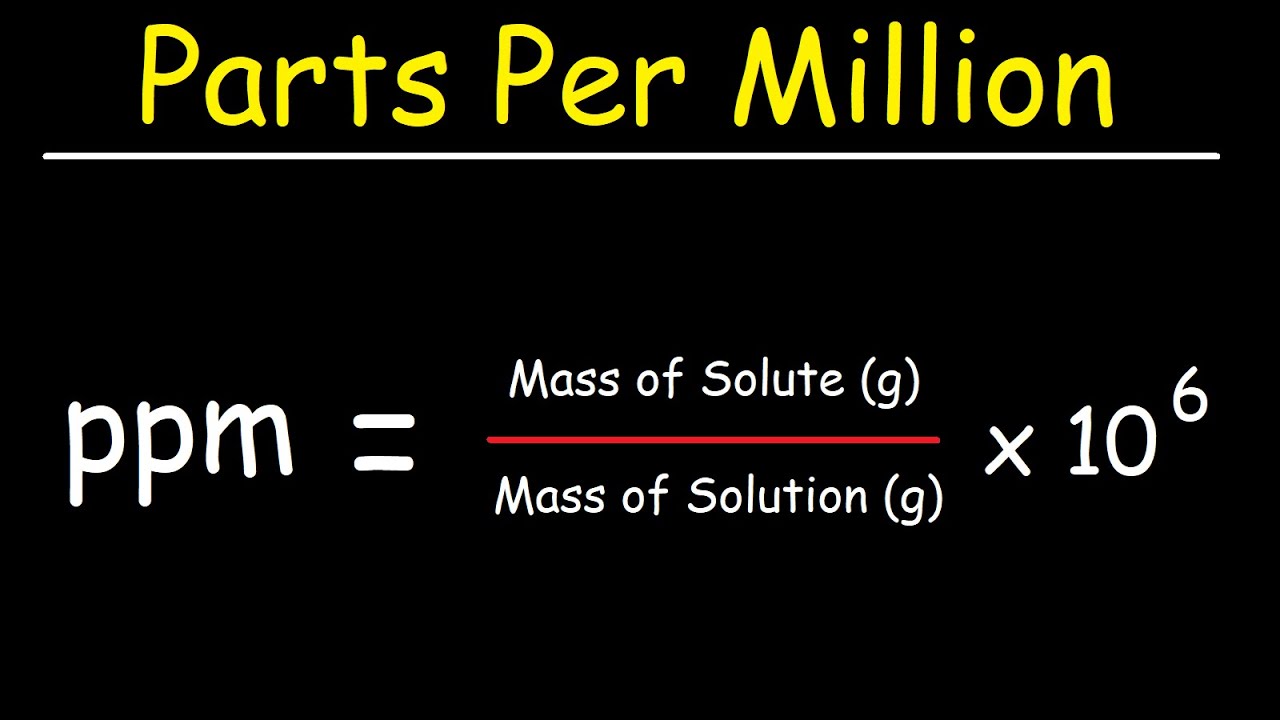
Photo Credit by: bing.com /