If you’re working on a chemistry project, you know that accuracy is key. But how do you account for uncertainty in your measurements? Calculating percentage uncertainty in chemistry is an essential skill that every chemist should know.
Whether you’re working in a lab or completing a chemistry assignment, calculating percentage uncertainty can be a frustrating and confusing process. It can be challenging to know where to start, how to account for different types of uncertainty, and how to apply the correct formula.
Calculating percentage uncertainty is the process of determining the degree of uncertainty in a measurement, expressed as a percentage of the measured value. This calculation is essential in chemistry because all measurements have some degree of uncertainty, and only accurate measurements can lead to valid conclusions about a chemical reaction’s nature.
In this article, we’ll go over the basics of how to calculate percentage uncertainty in chemistry. We’ll cover different types of uncertainty, how to calculate percentage uncertainty, and why it’s crucial to calculate this value in all your chemistry experiments.
Personal Experience of How to Calculate Percentage Uncertainty Chem
When I first started working with chemistry experiments, calculating percentage uncertainty was one of the most confusing concepts for me to grasp. I struggled to differentiate between the different types of uncertainty and to apply the correct formula to my results.
But after spending some time practicing and understanding the basics, I found that calculating percentage uncertainty became more comfortable and less of a challenge for me.
Types of Uncertainty in Chemistry
Before we dive into calculating percentage uncertainty, let’s first review the different types of uncertainty that you might encounter in your chemistry experiments.
Random uncertainty is the natural variation that occurs when measuring a property. This type of uncertainty may be due to instrument limitations or variations in the environment. It can be minimized by taking more measurements and calculating the average value.
Systematic uncertainty is associated with errors inherent in the measuring instrument. This error can be consistently overestimating or underestimating the true value.
Human error can also affect uncertainty. Mistakes made while taking measurements, noting down readings, or performing calculations can create uncertainty in the final measurement.
How to Calculate Percentage Uncertainty Chem
To calculate percentage uncertainty, you need to know the absolute uncertainty and the measured value. The absolute uncertainty is the uncertainty in the measurement, and it represents the degree of error in the measurement. The measured value is the actual value that you obtained in your experiment.
The formula for calculating percentage uncertainty is as follows:
% Uncertainty = (Absolute uncertainty / Measured Value) x 100%
For example, let’s say you measured the mass of a beaker to be 50.2 g, with an absolute uncertainty of ±0.1 g. Using the formula above, we can calculate the percentage uncertainty as follows:
% Uncertainty = (0.1 g / 50.2 g) x 100% = 0.2%
So the percentage uncertainty in this case is 0.2%.
Why is Calculating Percentage Uncertainty Chem Important?
Calculating percentage uncertainty is essential in chemistry because it allows you to determine the accuracy and precision of your measurements. When you’re working with chemical reactions, it’s essential to know the degree of error in your results to ensure that your conclusions are valid.
Furthermore, calculating percentage uncertainty can help you identify areas where you can improve the accuracy of your measurements. By minimizing uncertainty, you can obtain more accurate results, leading to more valid experiments and more in-depth insights.
Personal Experience of How to Reduce Uncertainty in Chemistry
One way that I found useful in reducing uncertainty in my chemistry experiments was to ensure that I took enough measurements to minimize random uncertainty. By taking several measurements and calculating the average value, I could obtain a more accurate result.
I also made sure that I carefully noted down readings and performed calculations correctly to avoid any potential human errors.
Frequently Asked Questions on How to Calculate Percentage Uncertainty Chem
Q1. What is absolute uncertainty?
Absolute uncertainty is the uncertainty in a measurement, and it represents the degree of error in the measurement.
Q2. How can I minimize random uncertainty?
You can minimize random uncertainty by taking more measurements and calculating the average value.
Q3. Why is calculating percentage uncertainty important?
Calculating percentage uncertainty is important because it allows you to determine the accuracy and precision of your measurements. This information is essential to ensure that your conclusions are valid.
Q4. How can I reduce uncertainty in my chemistry experiments?
You can reduce uncertainty by taking enough measurements to minimize random uncertainty, being careful when noting down readings and performing calculations, and ensuring that your measuring instruments are working correctly.
Conclusion of How to Calculate Percentage Uncertainty Chem
Calculating percentage uncertainty in chemistry is an essential skill that every chemist should know. By following the steps outlined in this article, you’ll be able to determine the degree of error in your measurements and obtain more accurate results. Remember that minimizing uncertainty is essential for obtaining valid results and deeper insights into chemical reactions.
Gallery
11.1 State Uncertainties As Absolute And Percentage Uncertainties [SL

Photo Credit by: bing.com / percentage chemistry absolute uncertainties ib
How To Calculate Percentage Uncertainty In Chemistry
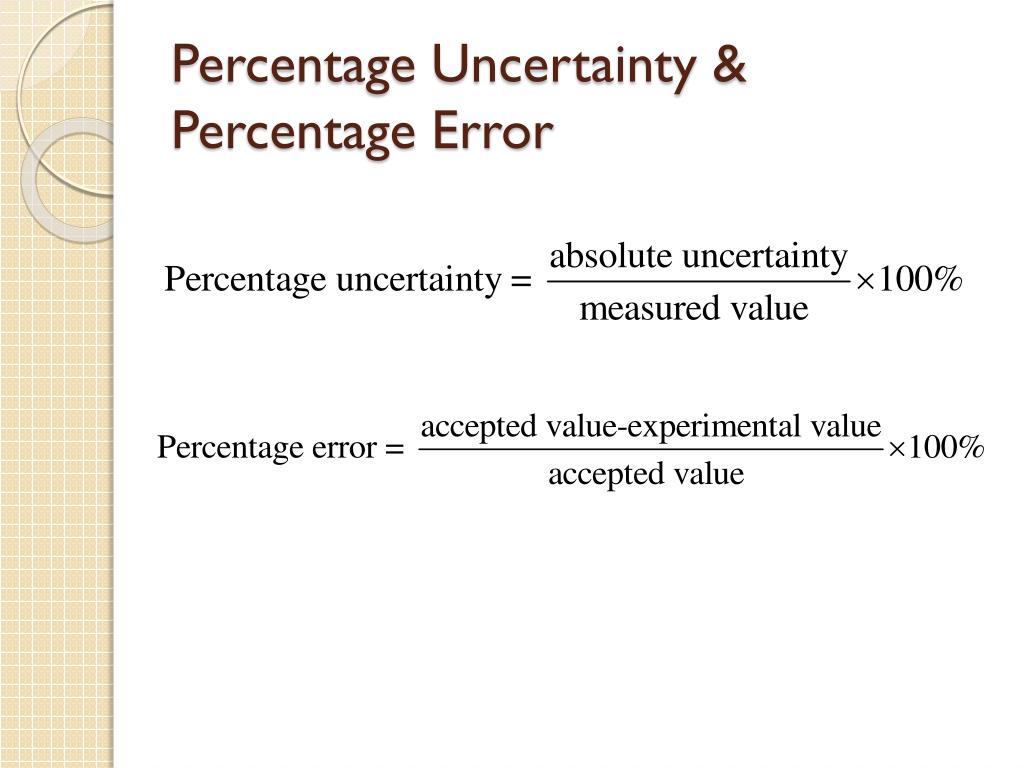
Photo Credit by: bing.com / uncertainty percentage calculating chemistry calculator uncertainties calculations
Percentage Uncertainty Chemistry Equation – Howto Diy Today
Photo Credit by: bing.com /
Percentage Uncertainty Chemistry Equation – Howto Diy Today
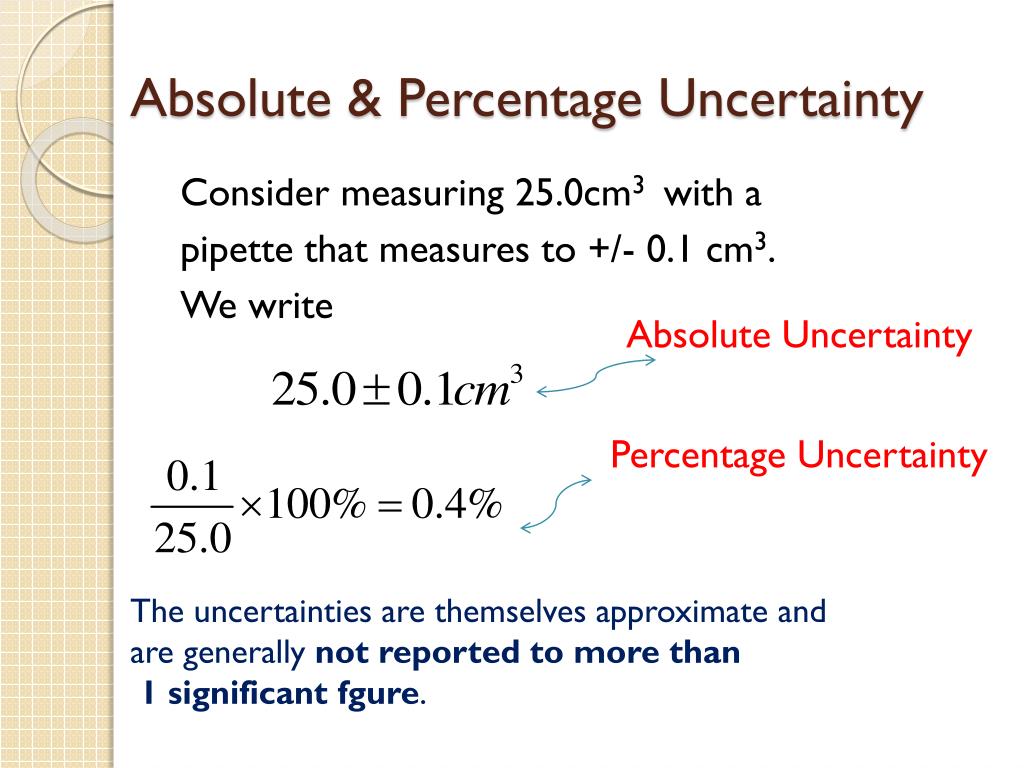
Photo Credit by: bing.com /
Convert Percent Uncertainty To Absolute : How To Calculate Uncertainty
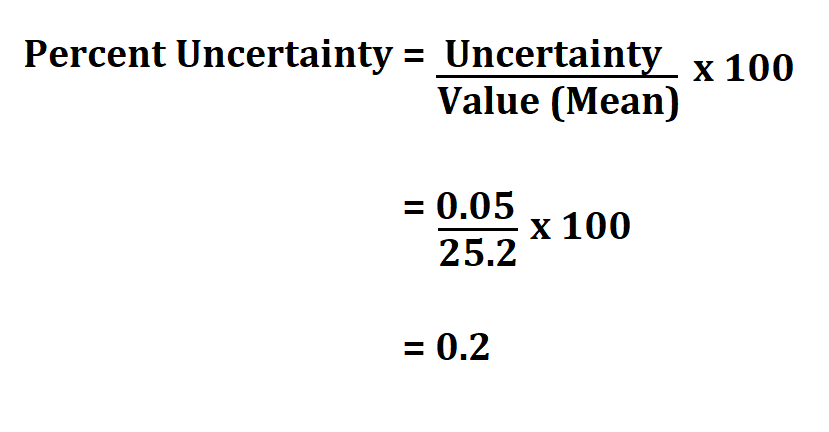
Photo Credit by: bing.com / uncertainty chemistry ib learntocalculate spelling