Chemistry experiments often involve multiple steps and calculations, one of which is calculating percentage yield. Whether you are a student learning about chemistry for the first time or a chemistry professional in need of a quick refresher, understanding how to calculate percentage yield is essential. In this post, we will explain how to calculate percentage yield in chemistry and provide some helpful tips along the way.
Pain Points
Calculating percentage yield can be a difficult task for beginners in chemistry. It requires a solid understanding of theoretical yield and actual yield, which is a concept that may be new to some. Additionally, small errors in measurements and calculations can quickly compound, leading to inaccurate results. These challenges can cause frustration and confusion for those attempting to determine the percentage yield of their experiments.
Answer
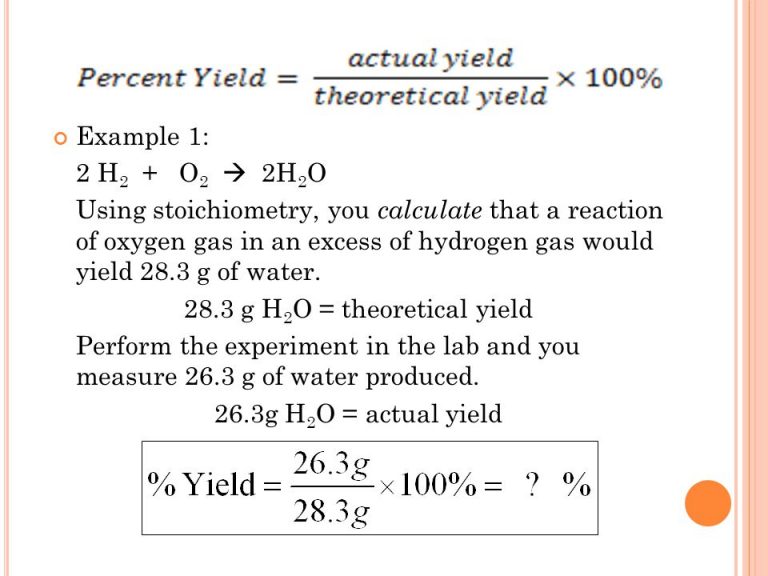
To calculate percentage yield, you need to first determine the theoretical yield and actual yield of your experiment. The theoretical yield is the maximum amount of product that should be produced based on the chemical equation, while actual yield is the amount of product you actually produced in the experiment. Once you have these values, divide the actual yield by the theoretical yield and multiply by 100 to get the percentage yield. The formula can be written as:

Summary
In summary, calculating the percentage yield of an experiment involves determining the theoretical yield and actual yield of the desired product, and then using a simple formula to find the percentage yield. Some common challenges to watch out for include inaccurate measurements and calculations, but by following the steps outlined above, one can accurately determine the percentage yield of their experiment.
How to Calculate Percentage Yield in Practice
When I was first learning how to calculate percentage yield in chemistry, I struggled with understanding the difference between theoretical yield and actual yield. It wasn’t until my lab partner explained that theoretical yield is the amount of product you would get if everything went perfectly according to the chemical equation, while actual yield is the amount you actually produced in the lab, that I was able to wrap my head around the concept. From there, it was simply a matter of plugging the values into the formula to get the percentage yield.

If you’re having trouble with the calculations, it’s always a good idea to double-check your measurements and make sure you’re using the right units. Additionally, don’t be afraid to ask a more experienced chemist for help or guidance. Ultimately, with practice and patience, you’ll master the art of calculating percentage yield.
Common Mistakes While Calculating Percentage Yield
One common mistake that people make when calculating percentage yield is forgetting to convert their measurements into the correct units. For example, if your theoretical yield is in grams but your actual yield is in milligrams, you’ll need to convert one of these values so that they are both in the same units before using the formula.

Another mistake that people make is using inaccurate measurements. It’s essential to be as precise as possible when measuring your reagents, as even slight variations in mass can have a significant impact on your results.
Further Explanation
When calculating percentage yield, it’s important to remember that the theoretical yield is always an ideal value that may not reflect the real-world conditions. It is based upon the assumption that the reaction will go to completion, with no side reactions, intermediate steps, or losses during the process. The actual yield, on the other hand, is the amount of product that you actually obtained through the reaction. It may be less than the theoretical yield due to various factors, including impurities or incomplete reactions.
Important Considerations
When calculating percentage yield, it’s important to keep in mind that the accuracy of your results depends on the accuracy of your measurements and calculations. As such, it’s always a good idea to double-check your work and ensure that your methodology is sound. Additionally, it’s helpful to know what factors may have impacted your results, so that you can make adjustments in future experiments.
Question and Answer
1. What is the formula for calculating percentage yield in chemistry?
The formula for calculating percentage yield in chemistry is actual yield divided by theoretical yield, multiplied by 100. It can be written as:

2. What is theoretical yield?
Theoretical yield is the maximum amount of product that can be produced according to the balanced chemical equation.
3. How do you ensure accurate measurements when calculating percentage yield?
It’s essential to use precise measurement tools and record measurements accurately. Additionally, double-checking measurements and conversions before using them in calculations is always a good idea.
4. What factors can impact the percentage yield of an experiment?
Several factors can impact the percentage yield of an experiment, including inaccuracies in measurements, incomplete reactions, and impurities in reagents.
Conclusion of How to Calculate Percentage Yield Chemistry
Calculating percentage yield is an essential skill for anyone conducting chemistry experiments. It involves determining the theoretical yield and actual yield of your experiment, and then using a simple formula to calculate the percentage yield. While there are some challenges and common mistakes to watch out for, with practice and patience, anyone can master the art of calculating percentage yield. Remember to be precise in your measurements and calculations, and to always understand the theoretical yield and actual yield before using the formula. Happy experimenting!
Gallery
Percent Yield Meaning : Definition Of Annual Percentage Yield – Math

Photo Credit by: bing.com / yield theoretical wikihow chemical equation equations chimica resa actual periodica rendement predict definitions lyfe
Percent Yield – Chemistry 101

Photo Credit by: bing.com / yield percent chemistry weebly
The Significance Of Percent Yield And Theoretical Yield Calculator

Photo Credit by: bing.com / theoretical
Percent Error Vs Percent Yield : Solved Conclusion Calculate The
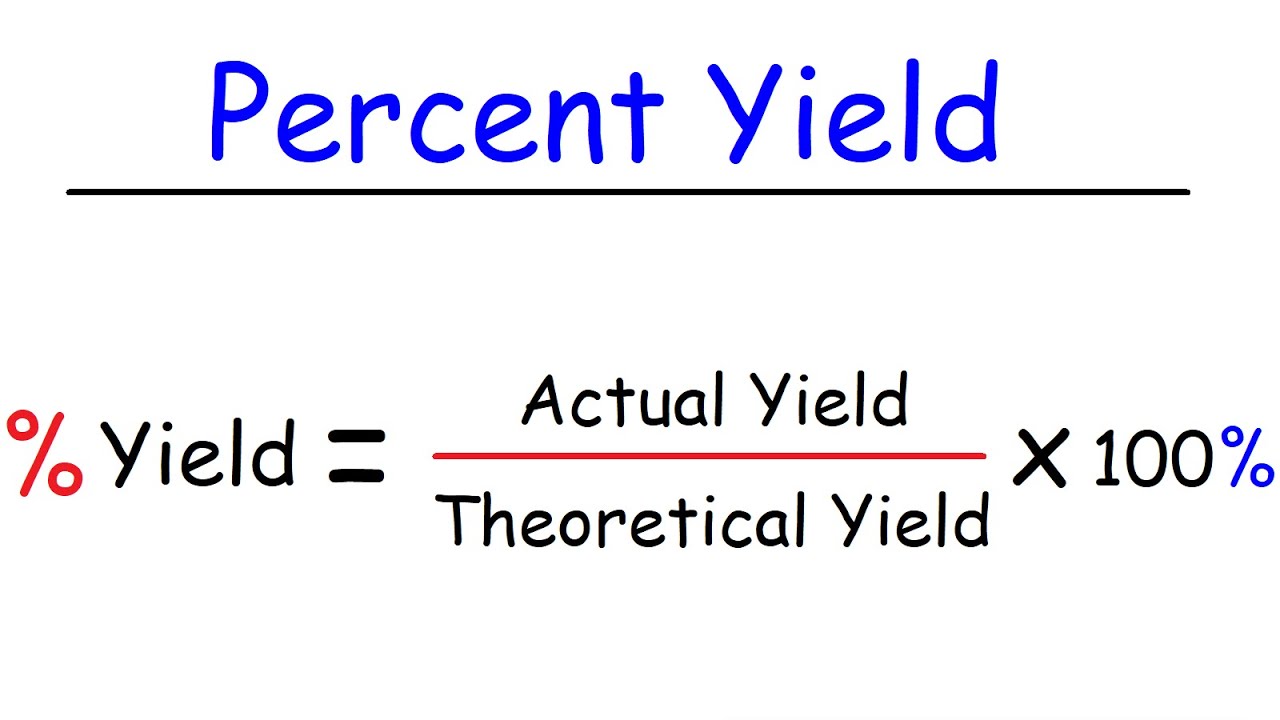
Photo Credit by: bing.com / yield percent calculate theoretical btown calculating calculator chegg
How To Calculate Percent Yield In Chemistry | Teaching Chemistry

Photo Credit by: bing.com / calculate chemistry theoretical formula reaction berekenen equations calculations experiment