In chemistry, calculating percentage yield is an important concept. It helps determine the amount of product produced from a chemical reaction, and if there are any losses in the process. By understanding how to calculate percentage yield, you can optimize chemical reactions and ensure maximum efficiency.
Many students struggle with the concept of percentage yield, and the calculation process can be confusing. Understanding the pain points of this topic can help you better explain and understand it.
To calculate percentage yield, you need to know the theoretical yield of a reaction, which is the maximum amount of product that can be produced based on the reactant amounts. You also need to know the actual yield, which is the amount of product actually produced in the lab. The percentage yield is calculated by dividing the actual yield by the theoretical yield, and multiplying by 100.
It’s important to note that actual yield is often less than theoretical yield due to losses during the reaction such as incomplete reactions, purification steps, or other unforeseen factors. By calculating percentage yield, you can determine the efficiency of the reaction and make adjustments to improve it in the future.
My Personal Experience with Calculating Percentage Yield
Back when I was a chemistry student, calculating percentage yield was one of the most confusing concepts. I struggled to understand what the theoretical yield was and how to calculate it. But once I got the hang of it, I found it incredibly useful to evaluate my lab work and determine the efficiency of a reaction. By optimizing the reaction conditions, I was able to improve the percentage yield and get better results.
Best Practices for Calculating Percentage Yield
It’s important to follow a few key best practices when calculating percentage yield. Firstly, make sure to record all measurements accurately and at the appropriate precision. Secondly, ensure that all reactants are of high purity and the reaction conditions are optimized for maximum yield. Finally, properly collecting and storing the product is crucial to prevent losses that could affect the actual yield.
Calculating Percentage Yield Step-by-Step
1. Determine the balanced chemical equation for the reaction

2. Calculate the theoretical yield using stoichiometry and the amount of limiting reagent

3. Conduct the reaction in the lab, and measure the actual yield

4. Calculate the percentage yield using the formula

Tips for Improving Percentage Yield
To improve the percentage yield of a reaction, there are a few steps you can take. Firstly, use high-purity reactants and carefully measure all quantities to minimize errors. Secondly, optimize reaction conditions such as temperature, pressure, and stirring to ensure complete reactions. Thirdly, properly purify and store the product to minimize losses. And finally, conduct multiple trials to establish an average yield and identify areas for improvement.
Questions and Answers
Q: What is the significance of calculating percentage yield in chemistry?
A: Percentage yield helps evaluate the efficiency of chemical reactions, and identify areas for improvement or optimization.
Q: What factors can cause the actual yield to be less than the theoretical yield?
A: There are various factors such as incomplete reactions, product losses during purification, or human error in measurements.
Q: How can you improve the percentage yield of a reaction?
A: You can improve percentage yield by using high-purity reactants, optimizing reaction conditions, properly purifying and storing the product, and conducting multiple trials to establish an average yield.
Q: Why is it important to accurately measure and record reactant quantities?
A: Accurately measuring and recording reactant quantities can minimize errors and ensure the theoretical yield is as accurate as possible.
Conclusion of How to Calculate Percentage Yield
Calculating percentage yield is an essential concept in chemistry that helps evaluate the efficiency of chemical reactions. By understanding the theoretical and actual yield of a reaction, you can calculate the percentage yield and identify areas for optimization. Following best practices such as accurately measuring reactants and optimizing reaction conditions can improve the percentage yield of a reaction. Hopefully, this post has helped clarify any confusion you may have had about calculating percentage yield and its importance.
Gallery
Percent Yield & Percent Purity (video Lessons, Examples And Solutions)

Photo Credit by: bing.com / yield percent formula purity example examples onlinemathlearning
Percent Error Vs Percent Yield : Solved Conclusion Calculate The
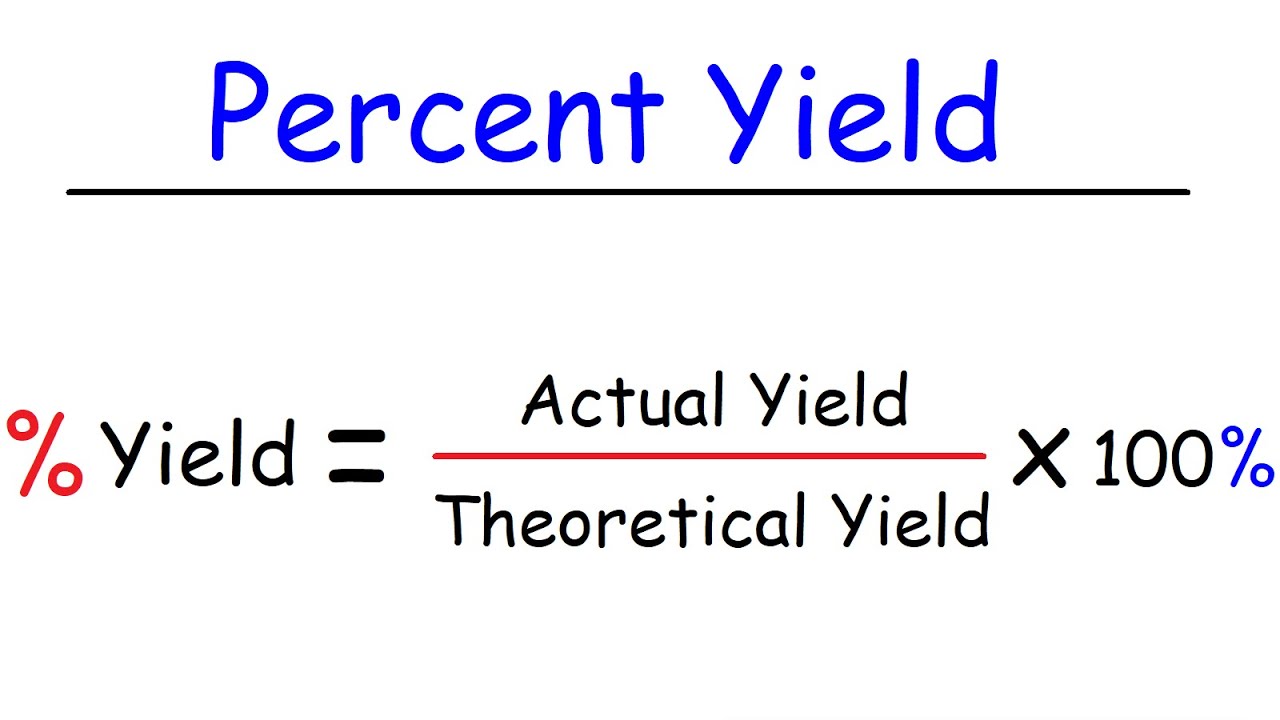
Photo Credit by: bing.com / yield percent calculate theoretical btown calculating calculator chegg
Calculating Percent Yield – YouTube

Photo Credit by: bing.com / calculating
The Significance Of Percent Yield And Theoretical Yield Calculator
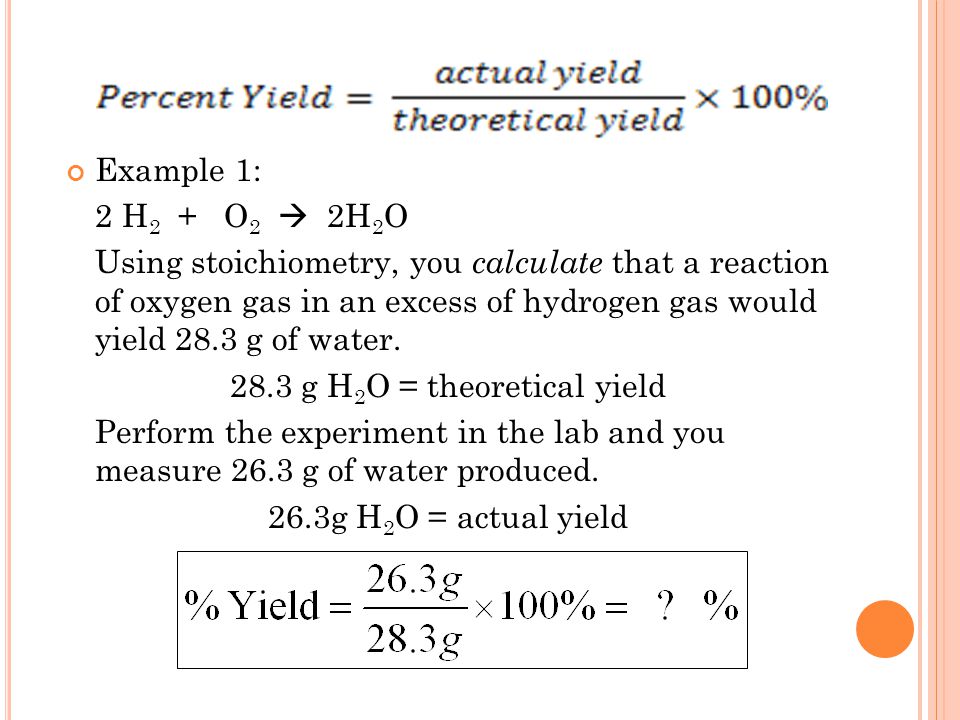
Photo Credit by: bing.com / yield theoretical significance provide
How To Calculate Percent Yield In Chemistry | Teaching Chemistry

Photo Credit by: bing.com / calculate chemistry theoretical formula reaction berekenen equations calculations experiment