Are you struggling with calculating percentage yield for your chemistry experiments? Well, fear not! In this article, we’ll break down the formula and offer tips to help you successfully calculate percentage yield.
As with many areas of chemistry, calculating percentage yield can be a confusing and frustrating process. Understanding the formula and being able to implement it correctly is essential for any successful experiment. However, many students and even professionals struggle with this concept and need help breaking it down into more manageable steps.
The formula for percentage yield involves taking the actual yield of the experiment and dividing it by the theoretical yield, then multiplying the result by 100. This will give you a percentage that represents the amount of product you were able to obtain compared to what you should have been able to produce.
To begin, you’ll need to determine the theoretical yield, which is the maximum amount of product that could be formed from the given reaction. This amount is determined by stoichiometry, which involves balancing the chemical equation and calculating the quantity of each reactant and product involved.
My Experience with Calculating Percentage Yield
When I first started conducting chemistry experiments, I struggled with calculating percentage yield. I would often get confused with the formula and end up with inaccurate results. However, after many trials and errors, I eventually learned the correct steps and techniques to accurately calculate percentage yield.
One tip I found helpful was to double-check my calculations and make sure I had the correct values before plugging them into the formula. I also learned how to properly balance chemical equations, which is essential for accurately determining theoretical yield.
Tips for Successfully Calculating Percentage Yield
If you’re struggling with calculating percentage yield, here are some tips to help you succeed:
1. Double-check your calculations.
Make sure you have the correct values before plugging them into the formula. This can prevent errors and ensure accurate results.
2. Properly balance the chemical equation.
Understanding stoichiometry and how to balance chemical equations is essential for accurately determining theoretical yield.
3. Use accurate measuring equipment.
Using precise measuring equipment can help ensure that you get an accurate actual yield measurement.
4. Be mindful of potential sources of error.
There are a variety of factors that can affect the accuracy of your results and lead to incorrect percentage yield calculations. Be sure to account for any potential sources of error, such as evaporation or impurities in your reactants.
Question and Answer
1. Q: What is the formula for calculating percentage yield?
A: The formula for percentage yield involves taking the actual yield of the experiment and dividing it by the theoretical yield, then multiplying the result by 100.
2. Q: How do you determine the theoretical yield?
A: The theoretical yield is determined by stoichiometry, which involves balancing the chemical equation and calculating the quantity of each reactant and product involved.
3. Q: What are some potential sources of error in determining percentage yield?
A: Potential sources of error can include evaporation, impurities in your reactants, and inaccurate measuring equipment.
4. Q: Why is it important to accurately calculate percentage yield?
A: Accurately calculating percentage yield is important for determining the efficiency of a reaction and making necessary adjustments for future experiments.
Conclusion of How to Calculate Percentage Yield Formula
While calculating percentage yield may initially seem overwhelming, with practice and attention to detail, it can become a manageable process. By following the formula and tips outlined in this article, you can ensure accurate results and greater success in your chemistry experiments.
Gallery
Percent Yield & Percent Purity (video Lessons, Examples And Solutions)

Photo Credit by: bing.com / yield percent formula purity example examples onlinemathlearning
How To Calculate Percent Yield In Chemistry | Teaching Chemistry

Photo Credit by: bing.com / yield theoretical equation calculator amount berekenen calculations
Percent Error Vs Percent Yield : Solved Conclusion Calculate The
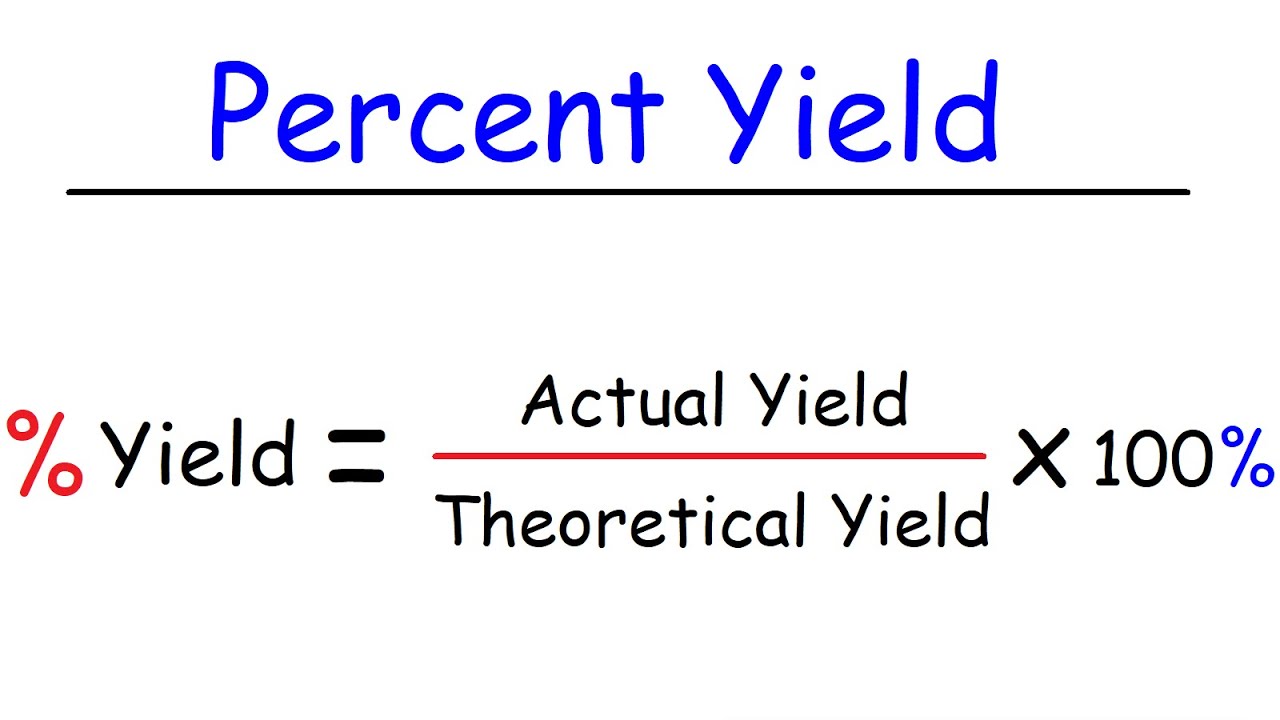
Photo Credit by: bing.com / yield percent calculate theoretical btown calculating calculator chegg
Molecular Formulas And Nomenclature
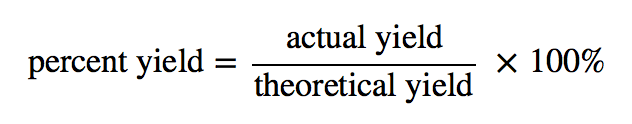
Photo Credit by: bing.com / yields yield chemistry percent theoretical actual formula example introductory chem formulas does
The Significance Of Percent Yield And Theoretical Yield Calculator
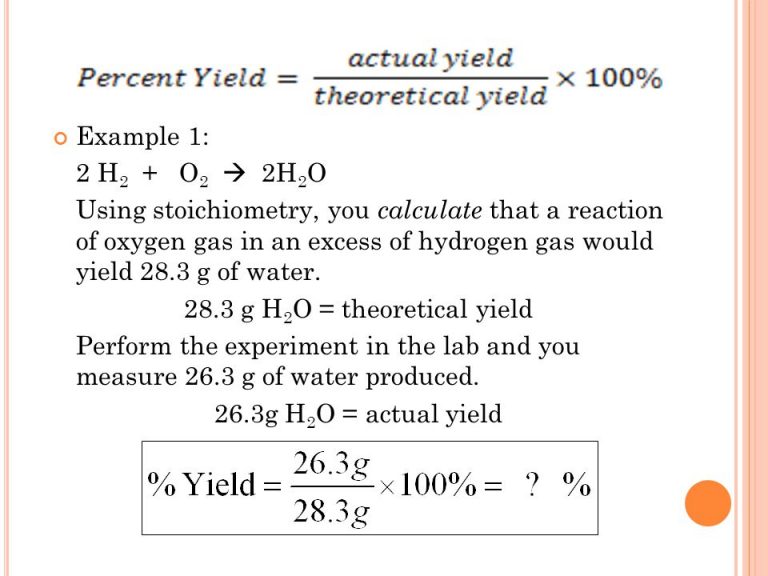
Photo Credit by: bing.com / theoretical