Have you ever wondered how to calculate percentage yield from mass? If you are a chemistry student, then you must know how important it is to determine the yield of a chemical reaction. The yield of a reaction shows how much product is formed from a given amount of reactant. Knowing the percentage yield can help scientists determine the efficiency of a reaction and whether there are any other variables impacting the process.
Calculating the percentage yield can be tricky for many students, especially if they are new to chemistry. Students may struggle with determining the theoretical yield and actual yield of a reaction. Additionally, measuring the mass of reactants and products accurately can also pose challenges for some students.
The formula to calculate percentage yield from mass is straightforward. It involves determining the theoretical yield of a reaction and the actual yield of the reaction. The theoretical yield is the maximum amount of product that can be produced in a reaction, while the actual yield is the amount of product that is actually produced from the reaction. Once you have these two values, you can use the following formula:
How to Calculate Percentage Yield from Mass
To calculate percentage yield from mass, you need to use the following formula:

Where:
- Percentage Yield (%) = The percentage of theoretical yield to actual yield.
- Actual Yield (g) = The amount of product formed during the reaction.
- Theoretical Yield (g) = The maximum amount of product that can be produced in the reaction, based on the number of moles of limiting reactant available.
Let’s say that you perform a chemical reaction, and the theoretical yield of the product is 15 g. However, you only obtain 10 g of product in the actual reaction. You can use the above formula to calculate the percentage yield:
Percentage Yield = Actual Yield / Theoretical Yield x 100
Percentage Yield = 10 g / 15 g x 100
Percentage Yield = 66.67%
Personal Experience with Calculating Percentage Yield from Mass
When I first started learning about how to calculate percentage yield from mass, I found it quite challenging. I struggled with determining the theoretical yield and the actual yield of a reaction. However, as I practiced more problems and got used to the formula, it became easier for me to determine the yield of a reaction. Now, I can confidently determine the efficiency of a reaction and identify any limitations that the experiment may have.
Tips for Calculating Percentage Yield from Mass
Here are a few tips to help you calculate percentage yield from mass:
- Measuring equipment should be accurate and precise in order to collect reliable data.
- Avoid errors by ensuring that each step of the calculation is performed accurately.
- Ensure that the reactants and products are pure and have not been contaminated.
- Always balance the chemical equations to avoid errors in calculation.
Why Understanding Percentage Yield from Mass Matters
Understanding how to calculate percentage yield from mass is important because it helps scientists determine the efficiency of a reaction. By knowing the yield, scientists can identify any limitations in the experiment and identify ways to optimize the reaction. This knowledge is essential in the chemical industry as it helps to minimize cost and maximize yield.
Conclusion
Calculating percentage yield from mass is an essential skill for chemistry students. The formula to calculate percentage yield from mass involves determining the theoretical yield and the actual yield of a reaction. By following the above tips, students can calculate percentage yield accurately and determine the efficiency of the reaction.
Question and Answer
Q. What is the significance of percentage yield in the chemical industry?
A. The percentage yield is important in the chemical industry because it helps to minimize cost and maximize yield. By knowing the yield, performance of the reaction can be improved by optimizing reaction conditions, reducing or avoiding wastage, and ensuring that product quality is maintained.
Q. How can one ensure that the reactants and products are pure and not contaminated?
A. To ensure that reactants and products are pure, they can be purified through distillation, recrystallization or other methods. In addition, an analytical technique such as the use of a GC-MS or HPLC can be employed to test for the purity of a compound before and after a reaction.
Q. How can errors be avoided when calculating percentage yield from mass?
A. To avoid errors when calculating the percentage yield, it is important to ensure that measuring equipment is accurate and precise. Additionally, each step of the calculation should be performed correctly. Finally, the reactants and products should be pure and balanced equations should be used to avoid errors in the calculation.
Q. How can one optimize the reaction based on the percentage yield?
A. One can optimize the reaction based on percentage yield by identifying the limitations in the experiment and identifying ways to improve the reaction. This can involve optimizing reaction conditions, such as temperature, pressure or pH, using more suitable catalysts or reagents, reducing wastage or identifying whether there are any impurities introduced during the reaction.
Gallery
Impressive Yield Of Reaction Calculator Write The Chemical Equation For

Photo Credit by: bing.com /
How To Calculate Percent Yield In Chemistry | Teaching Chemistry

Photo Credit by: bing.com / calculate chemistry theoretical formula reaction berekenen equations calculations experiment
The Significance Of Percent Yield And Theoretical Yield Calculator
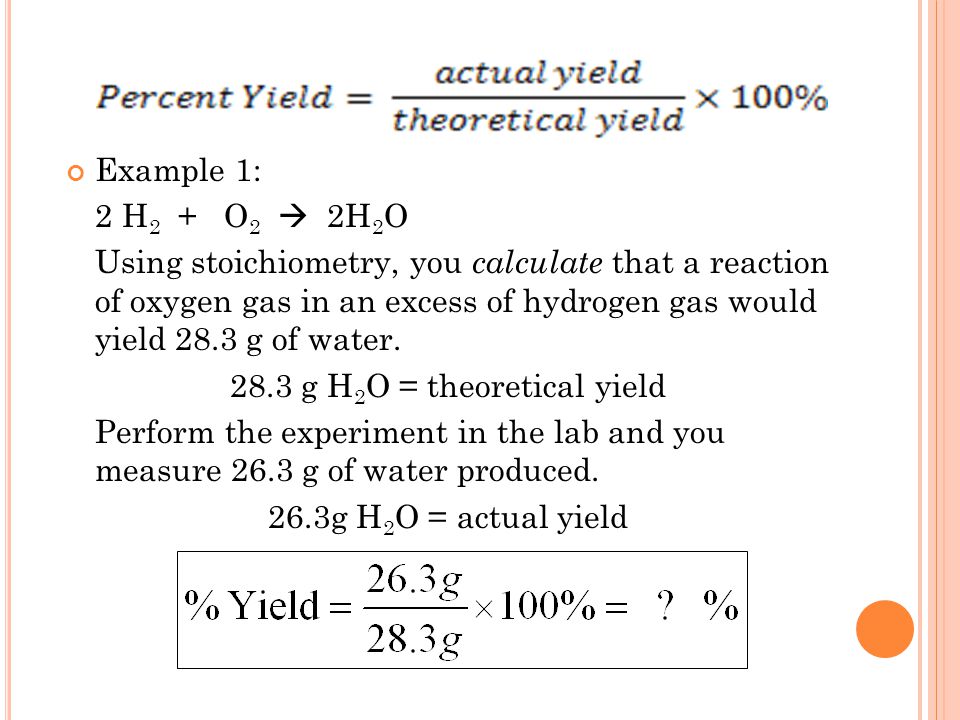
Photo Credit by: bing.com / yield theoretical significance provide
Percent Error Vs Percent Yield : Solved Conclusion Calculate The
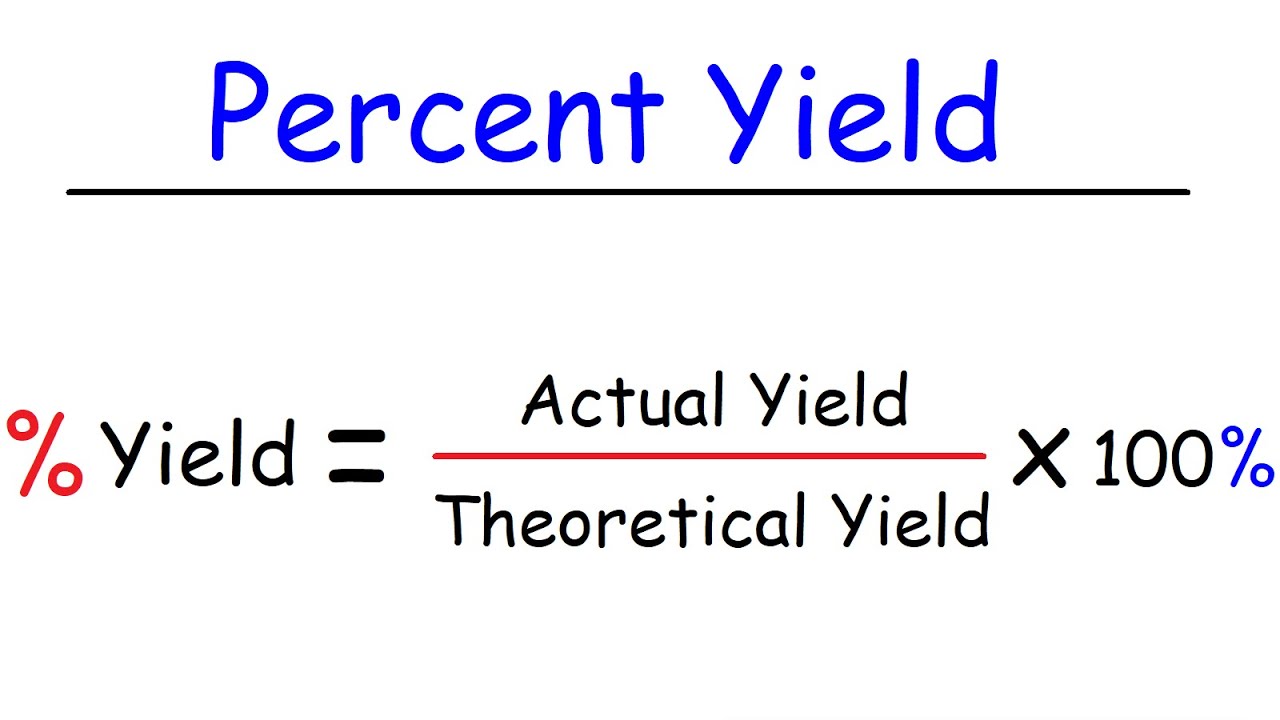
Photo Credit by: bing.com / yield percent calculate theoretical btown calculating calculator chegg
Percent Yield Meaning : How To Calculate Percent Yield In Chemistry 15

Photo Credit by: bing.com / calculate theoretical resa teorica aspirin reaction chemistry moles percentuale calcolare mole calcola mol gram