Have you ever wondered how to calculate percentage yield GCSE in your chemistry class? It can be a difficult concept to grasp, but it is an essential part of understanding chemical reactions. In this blog post, we will explore the ins and outs of calculating percentage yield GCSE and provide you with the knowledge to ace your next chemistry test.
Pain Points
Calculating percentage yield GCSE can be a source of frustration for many students. It requires an understanding of chemical equations and stoichiometry, which can be difficult to wrap your head around. Additionally, it can be challenging to determine the actual yield of a reaction, making it even more challenging to calculate the percentage yield.
Answering the Target
Percentage yield GCSE is calculated by dividing the actual yield of a reaction by the theoretical yield and multiplying by 100. The actual yield is the amount of product that is actually produced in a reaction, while the theoretical yield is the maximum amount of product that could be produced based on the amount of reactants used.
Main Points
To calculate percentage yield GCSE, you need to know the actual yield and the theoretical yield of a reaction. Once you have those values, you can plug them into the formula and calculate the percentage yield. It’s essential to note that the actual yield is always less than the theoretical yield, which means that the percentage yield is typically less than 100%. In addition to understanding the formula, it’s crucial to have a good grasp of stoichiometry and how it relates to chemical reactions.
Personal Experience with Percentage Yield GCSE
When I was first learning about how to calculate percentage yield GCSE, I struggled to understand the concept. I found it challenging to determine the actual yield of a reaction and was often confused about how to use stoichiometry to help in my calculations. However, with practice and guidance, I was able to grasp the concept and excel in my chemistry class.
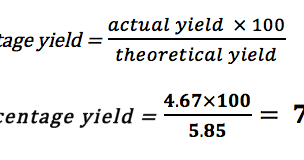
The Importance of Stoichiometry
Stoichiometry is an essential part of calculating percentage yield GCSE. It is the study of the relationships between the amounts of reactants and products in a chemical reaction. By using stoichiometry, you can predict the amount of product that will be produced based on the amount of reactants used. This information is crucial when calculating the theoretical yield of a reaction.
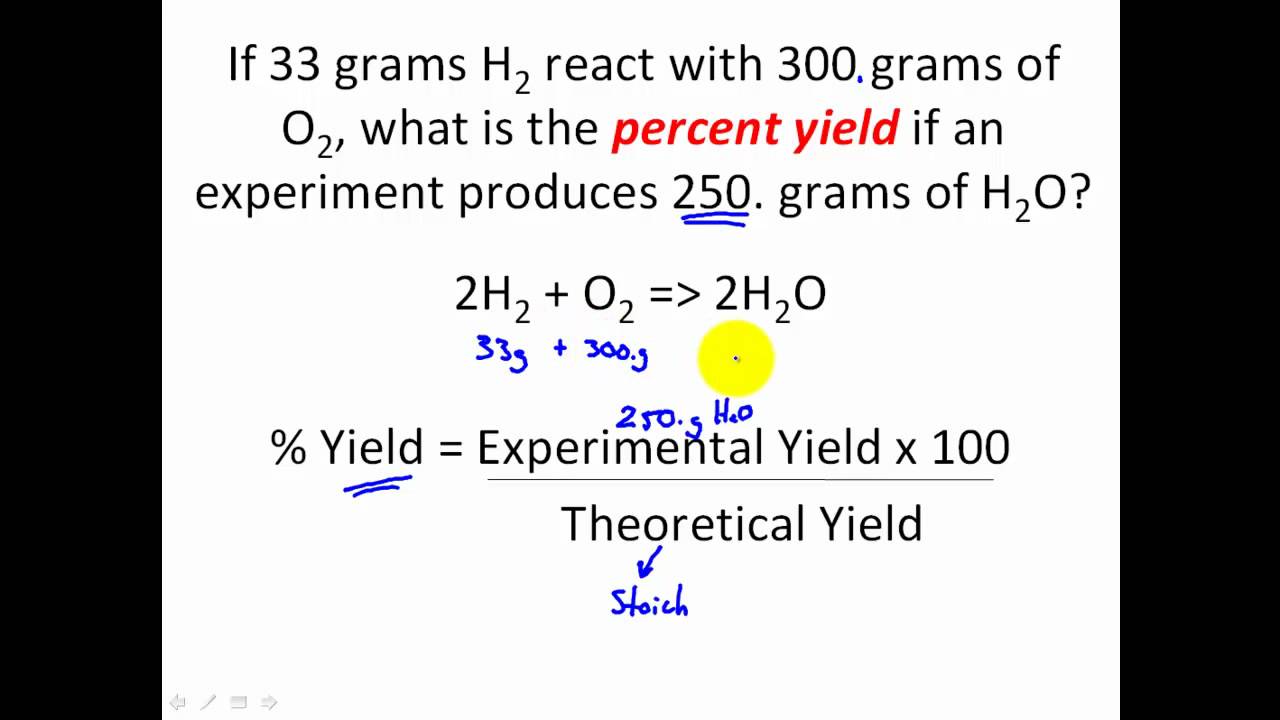
Finding the Actual Yield
Determining the actual yield of a reaction can be challenging. It requires precise measurements and can be affected by various factors, such as impurities in the final product or incomplete reactions. In most cases, the actual yield is determined through experimental means, such as weighing the final product or measuring the volume of gas produced.
Factors Affecting Percentage Yield
Several factors can affect the percentage yield of a reaction, including impurities in the reactants or products, incomplete reactions, and side reactions. It’s essential to take these factors into account when calculating the percentage yield of a reaction and to understand how they can impact the final result.
Applying the Formula
Now that you understand how to calculate percentage yield GCSE and the importance of stoichiometry and experimental measurements let’s apply the formula. Suppose you perform a reaction that produces 50 grams of product, and the theoretical yield of the reaction is 60 grams. To calculate the percentage yield, you would divide 50 by 60 and multiply by 100, which gives you a percentage yield of 83.3%.

Question and Answer
Q: What is percentage yield GCSE?
A: Percentage yield GCSE is the ratio of the actual yield of a reaction to the theoretical yield, multiplied by 100.
Q: Why is stoichiometry important in calculating percentage yield?
A: Stoichiometry is crucial in determining the amount of product that could be produced based on the amount of reactants used, which is needed to calculate the theoretical yield of a reaction.
Q: What factors can affect the percentage yield of a reaction?
A: Factors such as impurities in the reactants or products, incomplete reactions, and side reactions can all impact the percentage yield of a reaction.
Q: Why is calculating percentage yield GCSE important?
A: Calculating percentage yield GCSE is essential in determining the efficiency of a reaction and whether it is economical to perform the reaction on a large scale.
Conclusion of how to calculate percentage yield GCSE
Calculating percentage yield GCSE is an essential part of understanding chemical reactions. It requires an understanding of stoichiometry and experimental measurements and can be affected by various factors. By understanding the formula and the importance of stoichiometry, you can calculate the percentage yield of a reaction with accuracy and precision.
Gallery
Savvy-chemist: GCSE OCR Gateway Chemistry C5.1g-h Percentage And
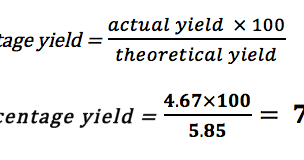
Photo Credit by: bing.com / gcse chemistry yield percentage theoretical
STOICHIOMETRY – Percent Yield Stoichiometry Problems – CLEAR & EASY

Photo Credit by: bing.com / stoichiometry problem solving chemistry yield percent problems solve easy gas
How To Calculate Percent Yield In Chemistry | Teaching Chemistry

Photo Credit by: bing.com / calculate chemistry theoretical formula reaction berekenen equations calculations experiment
Percent Error Vs Percent Yield : Solved Conclusion Calculate The
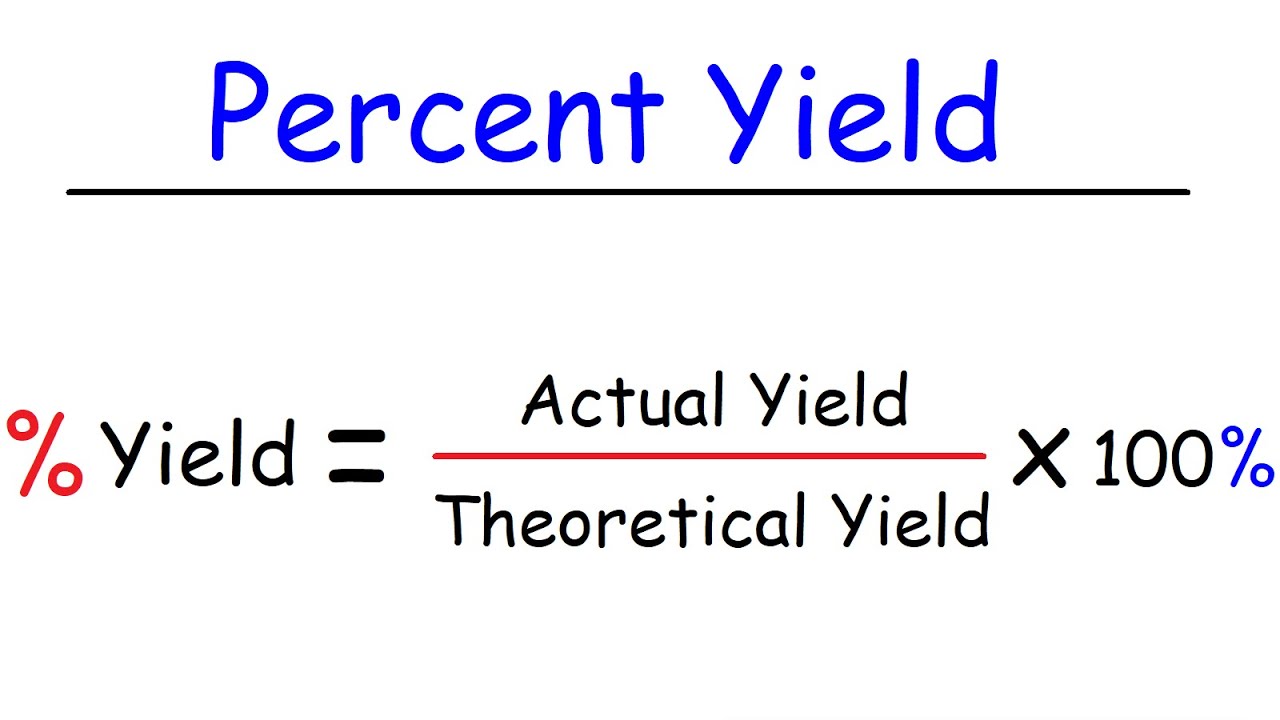
Photo Credit by: bing.com / yield percent calculate theoretical btown calculating calculator chegg
Percent Yield Meaning : Ch150 Chapter 6 Quantities In Chemistry

Photo Credit by: bing.com / yield purity ch150 onlinemathlearning quantities recovery