If you’re a chemistry student or researcher, you probably know the importance of calculating the percentage yield of a product after a chemical reaction. But do you know exactly how to do it? In this post, we’ll cover everything you need to know about how to calculate percentage yield of product.
When conducting chemical reactions, it’s important to know exactly how much product you can expect to yield. Without knowing this, you could end up wasting time and resources on reactions that don’t produce enough product. Additionally, calculating the percentage yield of a product can help you identify any inefficiencies in your reaction process.
The percentage yield of a product is the amount of final product obtained divided by the theoretical yield, multiplied by 100%. The theoretical yield is the amount of product that should be produced based on the reactants used in the reaction, assuming perfect conditions.
To calculate percentage yield of product, simply divide the actual yield (the amount of product you obtained in your reaction) by the theoretical yield, and multiply by 100%. This will give you the percentage yield of your reaction.
Overall, calculating the percentage yield of a product is crucial in the field of chemistry. By knowing the percentage yield of a reaction, you can optimize your experiments and identify any inefficiencies in your process.
My Experience with Calculating Percentage Yield of Product
When I was a chemistry student, I remember conducting a reaction that was highly inefficient. I was able to extract only a small amount of product from the reaction, and I couldn’t figure out what was going wrong. It wasn’t until I calculated the percentage yield of the reaction that I realized just how inefficient it was.
By calculating the percentage yield, I was able to identify that the reaction conditions were not ideal, and I was able to make some changes to optimize the reaction process. The next time I conducted the reaction, I was able to achieve a much higher percentage yield, and I saved myself a lot of time and resources in the process.
Tips for Calculating Percentage Yield of Product
When conducting a reaction, it’s important to be as precise as possible when measuring your reactants. Any deviation from the exact amounts required can greatly affect your percentage yield. Additionally, make sure you collect all of your product carefully and avoid any spills or losses. Finally, make sure you account for any impurities or byproducts that may have affected your yield.
Factors that Affect Percentage Yield of Product
Several factors can affect the percentage yield of a product, including the purity of reactants, reaction conditions, and the efficiency of separation techniques used to extract the product. Additionally, the stoichiometry of reactants (or the ratio of reactants used in the reaction) can greatly affect the theoretical yield and therefore the percentage yield of the reaction.
Common Mistakes when Calculating Percentage Yield of Product
One common mistake when calculating percentage yield of product is failing to account for impurities or byproducts. Additionally, using inaccurate measurements or not measuring reactants precisely enough can also greatly affect the calculation.
FAQs: How to Calculate Percentage Yield of Product
1. What does percentage yield mean?
Percentage yield refers to the amount of final product obtained in a reaction, relative to the theoretical yield.
2. Why is it important to calculate percentage yield of a product?
Calculating the percentage yield of a product is important because it can help you identify any inefficiencies in your reaction process, and optimize your experiments.
3. How do you calculate theoretical yield?
Theoretical yield can be calculated by using stoichiometry to determine the amount of product that would theoretically be produced based on the amount of reactants used in the reaction.
4. How do you increase percentage yield of a reaction?
To increase the percentage yield of a reaction, you can optimize your reaction conditions, improve the purity of your reactants, or use more efficient separation techniques to extract the product.
Conclusion of How to Calculate Percentage Yield of Product
Calculating the percentage yield of a product is an important step in any chemical reaction. By following best practices and accounting for all factors that can affect the yield, you can optimize your experiments and achieve higher yields of your desired product. Remember to always measure reactants precisely, collect product carefully, and account for any impurities or byproducts in your calculation.
Gallery
Percent Yield Meaning : How To Calculate Percent Yield In Chemistry 15

Photo Credit by: bing.com / calculate theoretical resa teorica aspirin reaction chemistry moles percentuale calcolare mole calcola mol gram
Percent Yield Meaning : Ch150 Chapter 6 Quantities In Chemistry

Photo Credit by: bing.com / yield purity ch150 onlinemathlearning quantities recovery
How To Calculate Percent Yield In Chemistry | Teaching Chemistry

Photo Credit by: bing.com / calculate chemistry theoretical formula reaction berekenen equations calculations experiment
Top Notch Percent Yield Of A Reaction Calculator Chemical Equilibrium
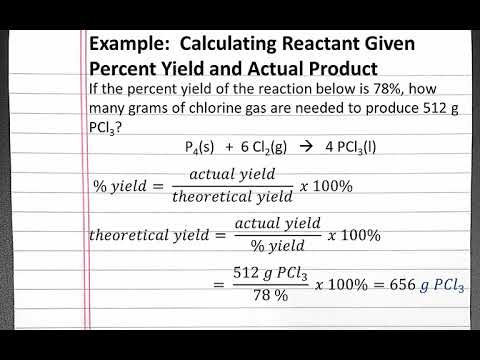
Photo Credit by: bing.com /
The Significance Of Percent Yield And Theoretical Yield Calculator
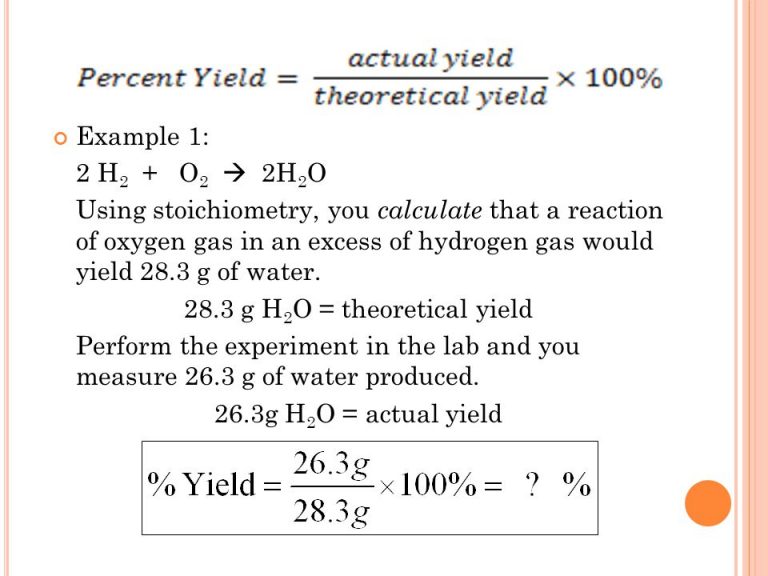
Photo Credit by: bing.com / theoretical